Abstract
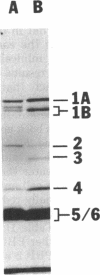
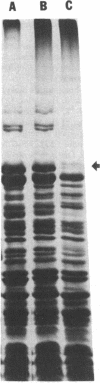
The penicillin-binding proteins (PBPs) found in the membranes of Escherichia coli X925 minicells (primarily cell ends or septa) were compared with those found in rod-shaped cells (primarily sidewalls) in an effort to determine whether certain PBPs are unevenly distributed over the bacterial cell membrane. The seven major PBPs of E. coli were all present in minicell membranes. PBP 1B was altered in minicells, however, appearing as two bands on sodium dodecyl sulfate-polyacrylamide gels rather than the usual three. PBP 2, which is needed for longitudinal growth of the cell but not for septum formation, was significantly reduced in minicell membranes. This observation is consistent with the fact that minicells contain very little sidewall material and raises the possibility that the specialized function of PBP 2 may be determined or regulated by its uneven topographical distribution in the membrane. None of the PBPs appeared to be selectively enriched in minicell membranes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buchanan C. E. Altered membrane proteins in a minicell-producing mutant of Bacillus subtilis. J Bacteriol. 1979 Jul;139(1):305–307. doi: 10.1128/jb.139.1.305-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Strominger J. L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. Solubilization and isolation of the membrane-bound DD-carboxypeptidase of Streptococcus faecalis ATCC9790. Properties of the purified enzyme. Eur J Biochem. 1978 Jul 17;88(1):297–305. doi: 10.1111/j.1432-1033.1978.tb12450.x. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Derepression of anthranilate synthase in purified minicells of Escherichia coli containing the Col-trp plasmid. J Bacteriol. 1973 Aug;115(2):615–622. doi: 10.1128/jb.115.2.615-622.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Production, properties and utility of bacterial minicells. Curr Top Microbiol Immunol. 1975;69:1–84. doi: 10.1007/978-3-642-50112-8_1. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Enzymes synthesizing and hydrolyzing murein in Escherichia coli. Topographical distribution over the cell envelope. Eur J Biochem. 1977 Nov 15;81(1):205–210. doi: 10.1111/j.1432-1033.1977.tb11942.x. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U., Teather R. M. Cell envelope composition of Escherichia coli K12: a comparison of the cell poles and the lateral wall. Eur J Biochem. 1974 Sep 16;47(3):567–572. doi: 10.1111/j.1432-1033.1974.tb03727.x. [DOI] [PubMed] [Google Scholar]
- Green E. W., Schaechter M. The mode of segregation of the bacterial cell membrane. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2312–2316. doi: 10.1073/pnas.69.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Tarpay M., Tomasz A. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Hirata S., Nakamuta S., Koike M. Inhibition of cell division of Escherichia coli by a new synthetic penicillin, piperacillin. Antimicrob Agents Chemother. 1978 Aug;14(2):257–266. doi: 10.1128/aac.14.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Goldman R., Tipper D. J., Feingold B., Strominger J. L. Morphology of an Escherichia coli mutant with a temperature-dependent round cell shape. J Bacteriol. 1978 Dec;136(3):1143–1158. doi: 10.1128/jb.136.3.1143-1158.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Strominger J. L. Simultaneous deletion of D-alanine carboxypeptidase IB-C and penicillin-binding component IV in a mutant of Escherichia coli K12. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2980–2984. doi: 10.1073/pnas.74.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachatourians G. G., Clark D. J., Adler H. I., Hardigree A. A. Cell growth and division in Escherichia coli: a common genetic control involved in cell division and minicell formation. J Bacteriol. 1973 Oct;116(1):226–229. doi: 10.1128/jb.116.1.226-229.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Tamaki S., Curtis S. J., Strominger J. L. Mutational evidence for identity of penicillin-binding protein 5 in Escherichia coli with the major D-alanine carboxypeptidase IA activity. J Bacteriol. 1979 Jan;137(1):644–647. doi: 10.1128/jb.137.1.644-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Nuchamovitz Y., Rozenhak S., Ron E. Z. Murein biosynthesis during a synchromous cell cycle of Escherichia coli B. J Bacteriol. 1978 May;134(2):458–461. doi: 10.1128/jb.134.2.458-461.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Regulation of murein biosynthesis and septum formation in filamentous cells of Escherichia coli PAT 84. J Bacteriol. 1977 Mar;129(3):1593–1600. doi: 10.1128/jb.129.3.1593-1600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Matsuhashi M., Mitsuhashi S. Comparative studies of penicillin-binding proteins in Pseudomonas aeruginosa and Escherichia coli. Eur J Biochem. 1979 Oct;100(1):41–49. doi: 10.1111/j.1432-1033.1979.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Nolan R. D., Hildebrandt J. F. Comparison of the penicillin-binding proteins of different strains of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1979 Sep;16(3):336–340. doi: 10.1128/aac.16.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara H., Horikawa S. Penicillin-binding proteins of Streptomyces cacaoi, Streptomyces olivaceus, and Streptomyces clavuligerus. Antimicrob Agents Chemother. 1980 Jan;17(1):1–7. doi: 10.1128/aac.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N. Mucopeptide biosynthesis by minicells of Escherichia coli. J Bacteriol. 1977 Jul;131(1):363–365. doi: 10.1128/jb.131.1.363-365.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E., Shepherd S. T., Chase H. A. Identification of the binding protein which may be the target of penicillin action in Bacillus megaterium. Nature. 1978 Feb 9;271(5645):568–570. doi: 10.1038/271568a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suginaka H., Blumberg P. M., Strominger J. L. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5279–5288. [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., van Heijenoort Y., Tamura T., Mizoguchi J., Hirota Y., van Heijenoort J. In vitro peptidoglycan polymerization catalysed by penicillin binding protein 1b of Escherichia coli K-12. FEBS Lett. 1980 Feb 11;110(2):245–249. doi: 10.1016/0014-5793(80)80083-4. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Membrane assembly in Escherichia coli. II. Segregation of preformed and newly formed membrane proteins into cells and minicells. Biochem Biophys Res Commun. 1971 Jul 16;44(2):503–509. doi: 10.1016/0006-291x(71)90630-9. [DOI] [PubMed] [Google Scholar]