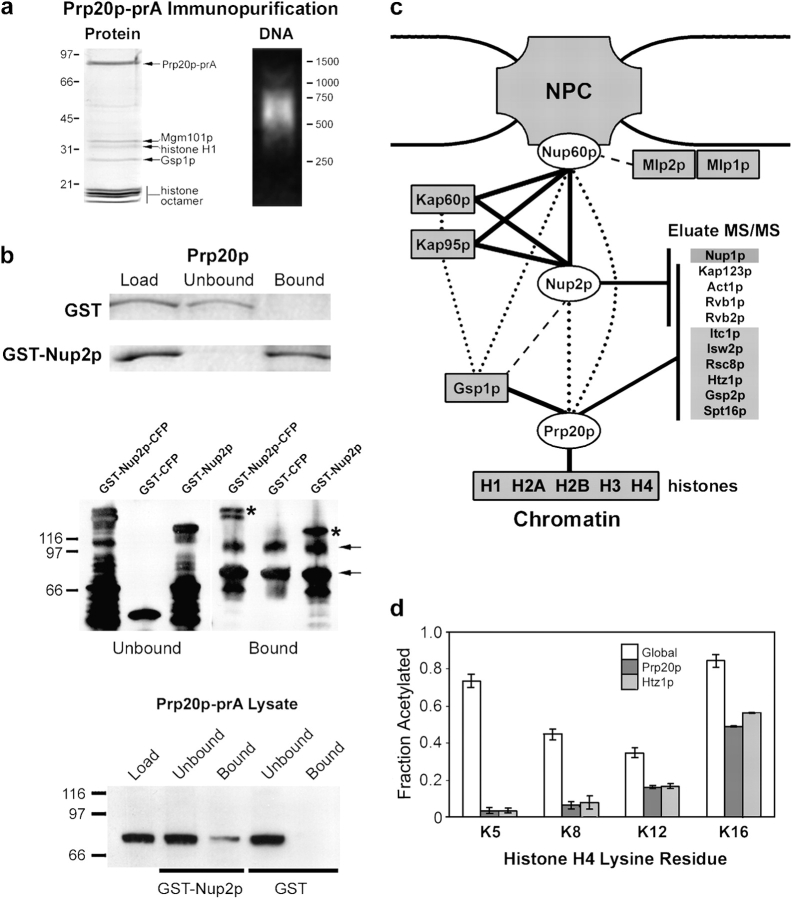
Figure 1.
Prp20p and Nup2p interact with chromatin remodeling factors. (a) Prp20p is nucleosome associated. (Left) Prp20p-prA was immunopurified from yeast whole-cell lysates and abundant copurifying proteins were identified by MS analysis of gel slices. Components of the histone octamer, H2A, H2B, H3, and H4, were present as well as the linker histone, H1 and Ran/Gsp1p. The presence of Mgm101p is not specific (see text). (Right) Prp20p-prA eluates contain DNA. Eluates were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining. Prp20p-prA associated DNA is ∼600 bp in length due to chromatin shearing during the cell lysis procedures. (b) The interaction between Nup2p and the Prp20p–nucleosome complex can be reconstituted in vitro. (Top) Bacterially expressed and purified Prp20p was incubated with glutathione resin coated with GST or GST-Nup2p. Unbound and bound proteins were resolved by SDS-PAGE and visualized with Coomassie blue. (Middle) The Prp20p–nucleosome complex was bound to IgG-coated magnetic beads and then incubated with bacterially expressed and purified GST-Nup2p-CFP, GST-CFP, or GST-Nup2p. Immunoblotting for GST revealed that although GST alone could not bind to the Prp20p–nucleosome complex, GST chimeras containing Nup2p bound efficiently (asterisks). Arrows indicate nonspecific immunoreactive proteins. (Bottom) Glutathione resin coated with GST-Nup2p, but not GST alone, was able to capture Prp20p-prA from yeast extracts. (c) Interactions between the NPC and chromatin. Proteins present at Coomassie blue–detectable levels in Nup2p, Nup60p, and/or Prp20p immunopurifications are connected by solid black lines. Other known physical and yeast two-hybrid interactions are shown by dotted and dashed lines, respectively (Dingwall et al., 1995; Rexach and Blobel, 1995; Denning et al., 2001; Feuerbach et al., 2002). High coverage tandem MS (MS/MS) of immunopurification eluates from Nup2p, Prp20p, and Nup60p,as well as Kap95p and Nup49p (as controls) was also performed. The inset list (Eluate MS/MS) shows proteins present exclusively in Nup2p eluates (top) or Prp20p eluates (bottom) and those present in both eluates (middle). (d) Nucleosomes associated with Prp20p and Htz1p possess unique acetylation patterns suggestive of boundary chromatin. The acetylation levels of residues K5, K8, K12, and K16 of histone H4 were quantified by mass spectrometry for global (white), Prp20p-associated (dark gray), and Htz1p-associated (light gray) nucleosomes.