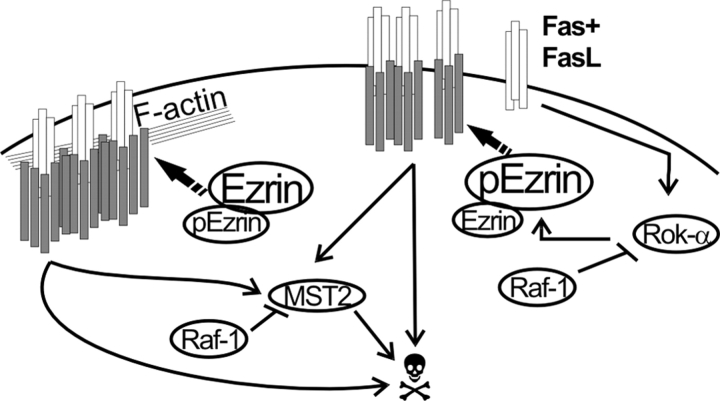
Figure 8.
Role of Raf-1 in Fas-mediated apoptosis: a working model. Fas binding to FasL stimulates DISC formation and internalization. Both of these processes depend on the linkage of Fas to the cytoskeleton. Phosphorylation of ezrin on T567 by Rok-α promotes Fas clustering but reduces DISC formation and internalization, generating a prolonged, albeit less efficient, Fas signal. In WT cells, formation of a Raf-1–Rok-α complex restrains Rok-α activity and ezrin phosphorylation. In addition, direct binding to Raf-1 prevents the dimerization and phosphorylation of the proapoptotic kinase MST-2 (O'Neill et al., 2004). In the absence of Raf-1, Rok-α activity and ezrin phosphorylation generate a prolonged Fas signal, boosted by unrestrained MST-2 stimulation. White rods, Fas; gray rods, DISC components.