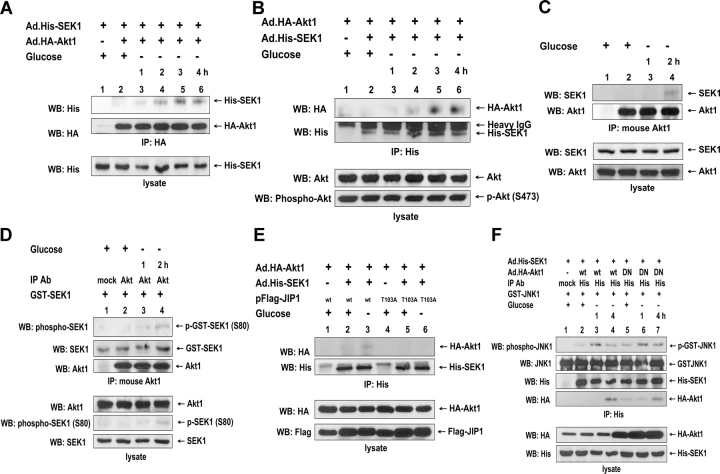
Figure 8.
Interaction between SEK1 and Akt1 and its role in SEK1 enzyme activity during glucose deprivation. DU-145 cells were coinfected with adenoviral vector containing His-tagged SEK1 (Ad.His-SEK1) and Ad.HA-Akt1 at an MOI of 10 (A, B, E, and F) and were transiently transfected with pFlag-JIP1-wt (wild type) or pFlag-JIP1–Thr-103A (mutant type; E). (A and B) After 48 h of incubation, cells were exposed to glucose-free medium for various times (1–4 h) and were lysed. Cell lysates were immunoprecipitated with anti-HA antibody (A) or anti-His antibody (B) and were immunoblotted with anti-His or anti-HA antibody (top). The presence of His-SEK1, Akt, or phospho-Akt in the lysates was verified by immunoblotting (bottom). (C and D) DU-145 cells were exposed to glucose-free medium for 1 or 2 h and were lysed. Lysates were immunoprecipitated with anti–mouse Akt antibody. (C) Immunoprecipitates were analyzed for SEK1 binding with anti-SEK1 or anti–rabbit Akt antibody (top). The presence of SEK1 and Akt in the lysates was verified by immunoblotting (bottom). (D) Immunoprecipitates were examined for the phosphorylation of SEK1 (Ser-80) by Akt. 0.5 μg GST-SEK1 was incubated with immunoprecipitated Akt in kinase buffer containing ATP at 30°C for 1 h. Phosphorylated proteins were resolved by SDS-PAGE and were analyzed by immunoblotting with anti–phospho-SEK1 (Ser-80) antibody. The presence of GST-SEK1 or Akt in the immunoprecipitate was verified by immunoblotting with anti-SEK1 antibody or anti–rabbit Akt antibody, respectively (top). The presence of Akt, phospho-SEK1, or SEK1 in the lysates was verified by immunoblotting (bottom). (E) After 48 h of incubation, cells were exposed to glucose-free medium for 4 h and were lysed. Lysates were immunoprecipitated with anti-His antibody and were immunoblotted with anti-HA or anti-His antibody (top). The presence of HA-Akt1, Flag-JIP1 wild type, or Flag-JIP1–Thr-103A in the lysates was verified by immunoblotting (bottom). (F) DU-145 cells were coinfected with Ad.His-SEK1 and Ad.HA-Akt1 wild type (wt) or Ad.HA-Akt1 dominant negative mutant type (DN) at an MOI of 10. After 48 h of incubation, cells were exposed to glucose-free medium for 1 or 4 h and were lysed. Lysates were immunoprecipitated with anti-His antibody. To examine the catalytic activity of SEK1, 0.5 μg GST-JNK1 was incubated with immunoprecipitated His-SEK1 in kinase buffer containing ATP at 30°C for 1 h. Phosphorylated proteins were resolved by SDS-PAGE and were analyzed by immunoblotting with anti–ACTIVE JNK antibody. The presence of GST-JNK1, His-SEK1, or HA-Akt1 in the immunoprecipitates was verified by immunoblotting (top). The presence of HA-Akt1 (wt, DN) or His-SEK1 in the lysates was verified by immunoblotting (bottom).