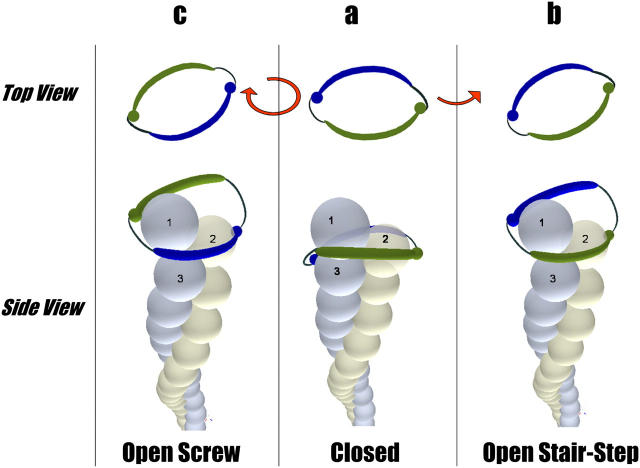
Figure 1.
Two modes of processive capping of actin filaments by a dimer of formin homology domain FH2. The model is based on the structure of an FH2–formin complex that was established crystallographically (Otomo et al., 2005). Spheres represent the actin monomers. The formin bridges are shown as blue and green elongated bodies winding around the actin filament. Red arrows indicate the directions of FH2 rotation with respect to the filament bulk. (a) The closed state of the formin–actin complex, which is unavailable for insertion of new actin monomers. The green bridge binds the protruding (actin 1) and penultimate (actin 2) subunits, whereas the second, blue bridge binds actins 2 and 3 subunits. (b) The stair-stepping mode of processive capping. The blue bridge migrates from actins 2 and 3 to actin 1 and exposes its post domain for insertion of a new actin monomer. The FH2 dimer rotates by ∼14° in the direction of twist of the long-pitch actin helix. (c) The screw mode of processive capping. The two bridges undergo a screwlike motion around the filament until they bind in the new position corresponding to the open state of the filament end. The FH2 dimer rotates in the direction of the short-pitch actin helix, which is opposite to the rotation direction of the stair-stepping mode. Rotation angle in the screw mode is approximately −166°.