Figure 3.
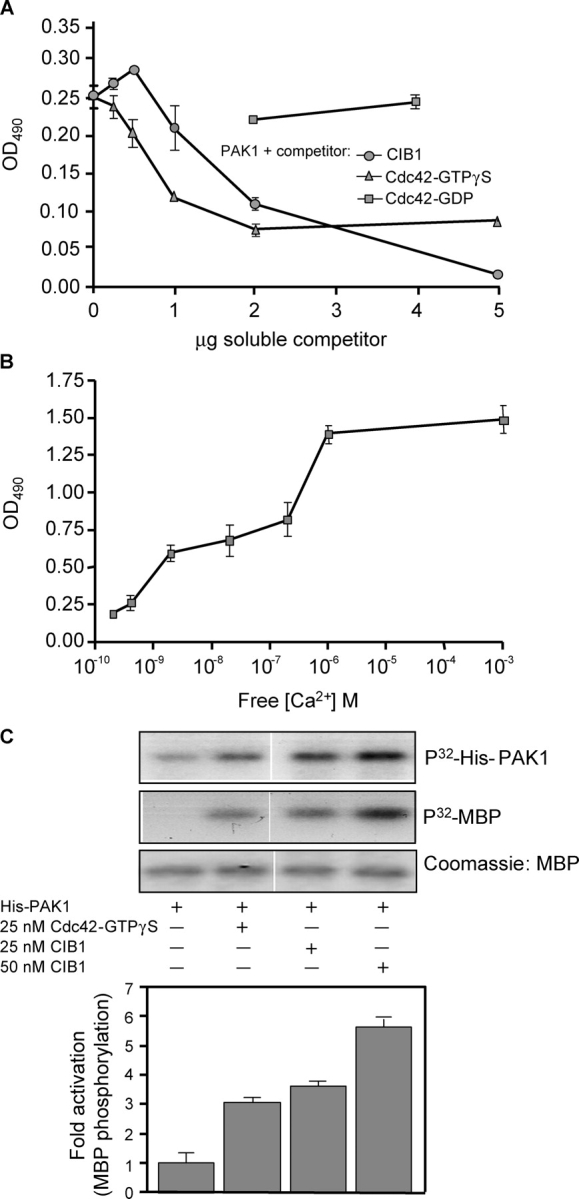
Cdc42 and Ca 2+ affect the CIB1–PAK interaction, and CIB1 stimulates PAK1 activity in vitro. (A) Activated Cdc42-GTPγS or CIB1, but not inactive Cdc42-GDP, competes with immobilized CIB1 for binding to soluble His-PAK1. Soluble His-PAK1 was incubated with increasing concentrations of soluble CIB1 or Cdc42 that was preloaded with GDP or GTPγS before incubation with immobilized CIB1. (B) Determination of Ca2+-dependent binding of His-PAK1 to CIB1. His-PAK1 was diluted in buffer containing 0–5 mM EGTA before addition to immobilized CIB1. Approximate free Ca2+ concentrations were calculated using the MaxChelator program (Bers et al., 1994). (C) Stimulation of recombinant His-PAK1 activity by recombinant CIB1 or Cdc42-GTPγS. His-PAK1 autophosphorylation was assayed in the absence (top) or presence of myelin basic protein (MBP) to detect active kinase (middle). White lines indicate that intervening lanes have been spliced out. Myelin basic protein phosphorylation ([32P]MBP) from densitometry analysis induced by His-PAK1 alone was assigned a value of 1 (bar graph). Data represent means ± SEM (error bars; n = 3).
