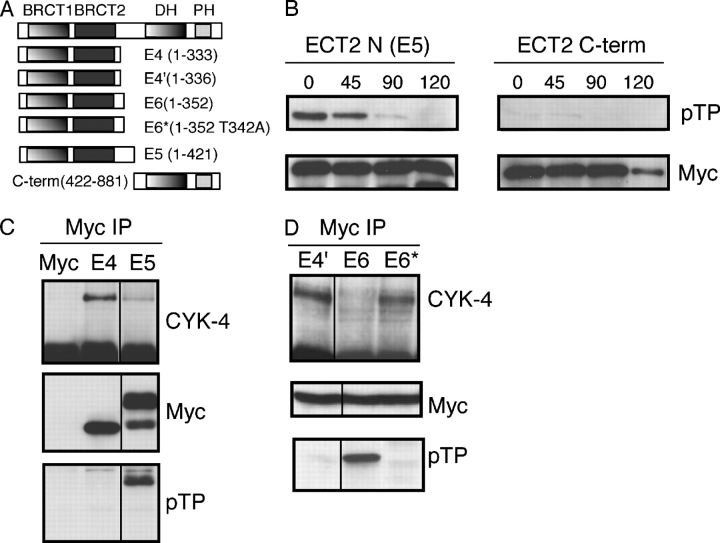
Figure 9.
Identification of a phosphorylation site that regulates the CYK-4–ECT-2 interaction. (A) Schematic of the ECT2 constructs used. (B) The NH2- and COOH-terminal halves (E5 and C-term) of ECT2 were transfected into HeLa cells, which were then arrested in metaphase with nocodazole. Cells were released from the block and collected at the indicated times. The myc-tagged ECT2 constructs were immunoprecipitated and Western blotted with anti-myc and anti–phospho-Thr-Pro antibodies. (C) ECT2 fragments are differentially competent to bind CYK-4 during metaphase. The differential association inversely correlates with reactivity with anti–phospho-Thr-Pro antibody. ECT2 constructs were transfected into HeLa cells, which were then arrested in metaphase with nocodazole. Lysates were prepared and the myc-tagged ECT2 constructs were immunoprecipitated and Western blotted with anti-CYK-4, anti-myc, and anti–phospho-Thr-Pro antibodies. (D) Identification of threonine 342, which, when mutated to alanine, prevents TP phosphorylation and allows ECT2 to bind CYK-4 during metaphase. This experiment was performed as described in C.