Abstract
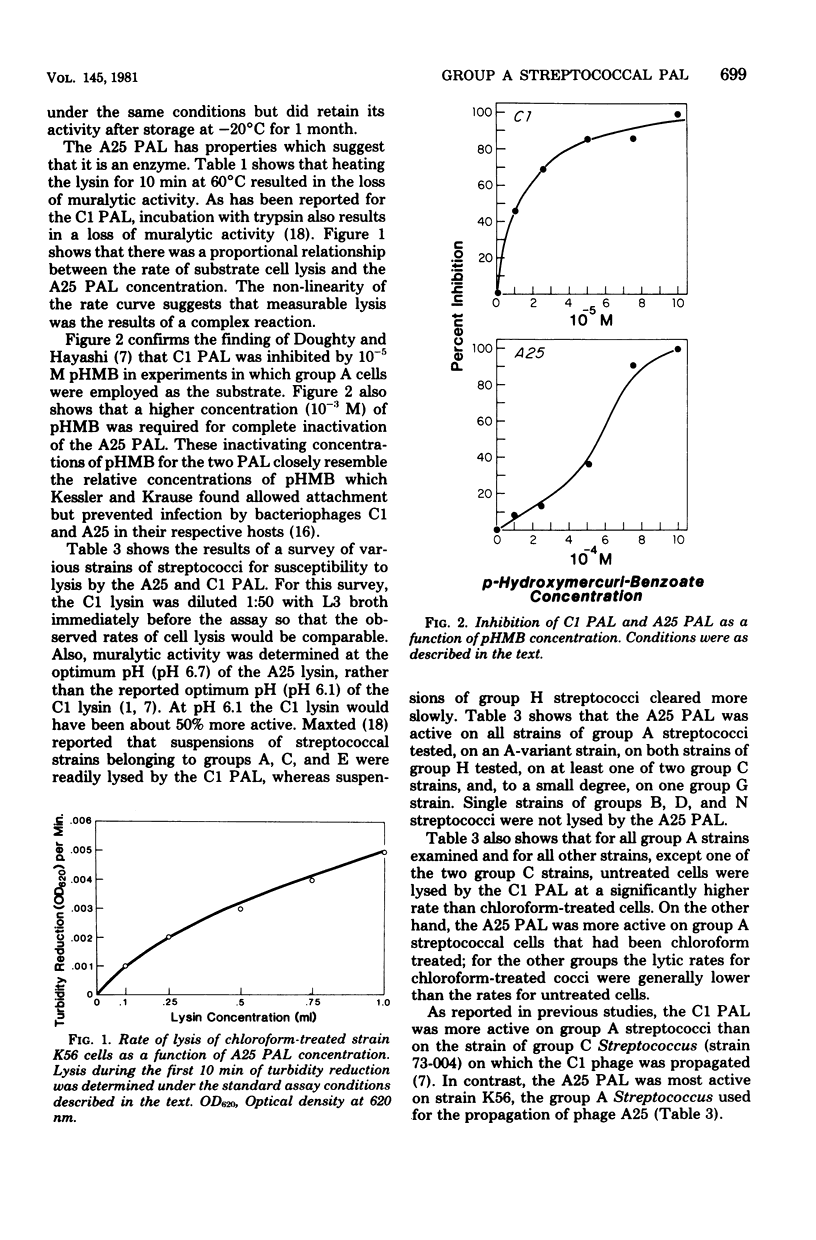
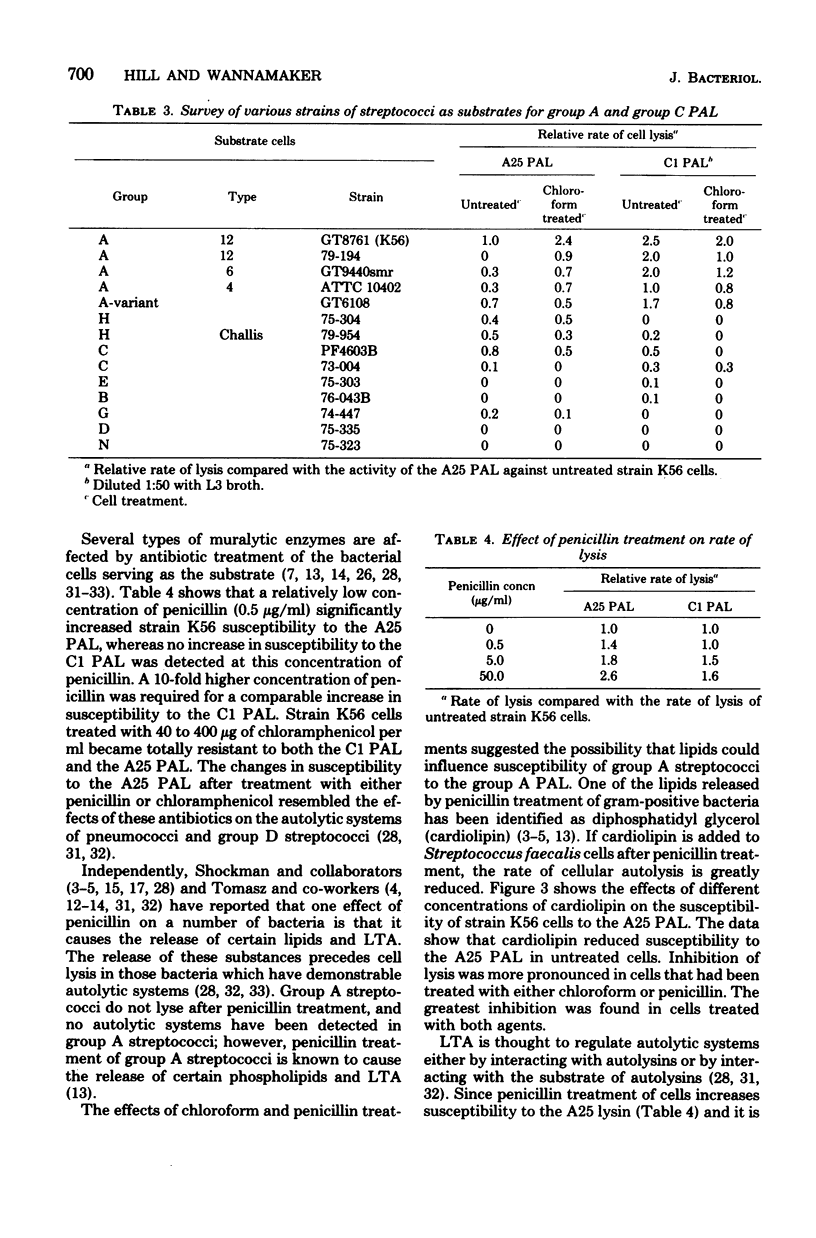
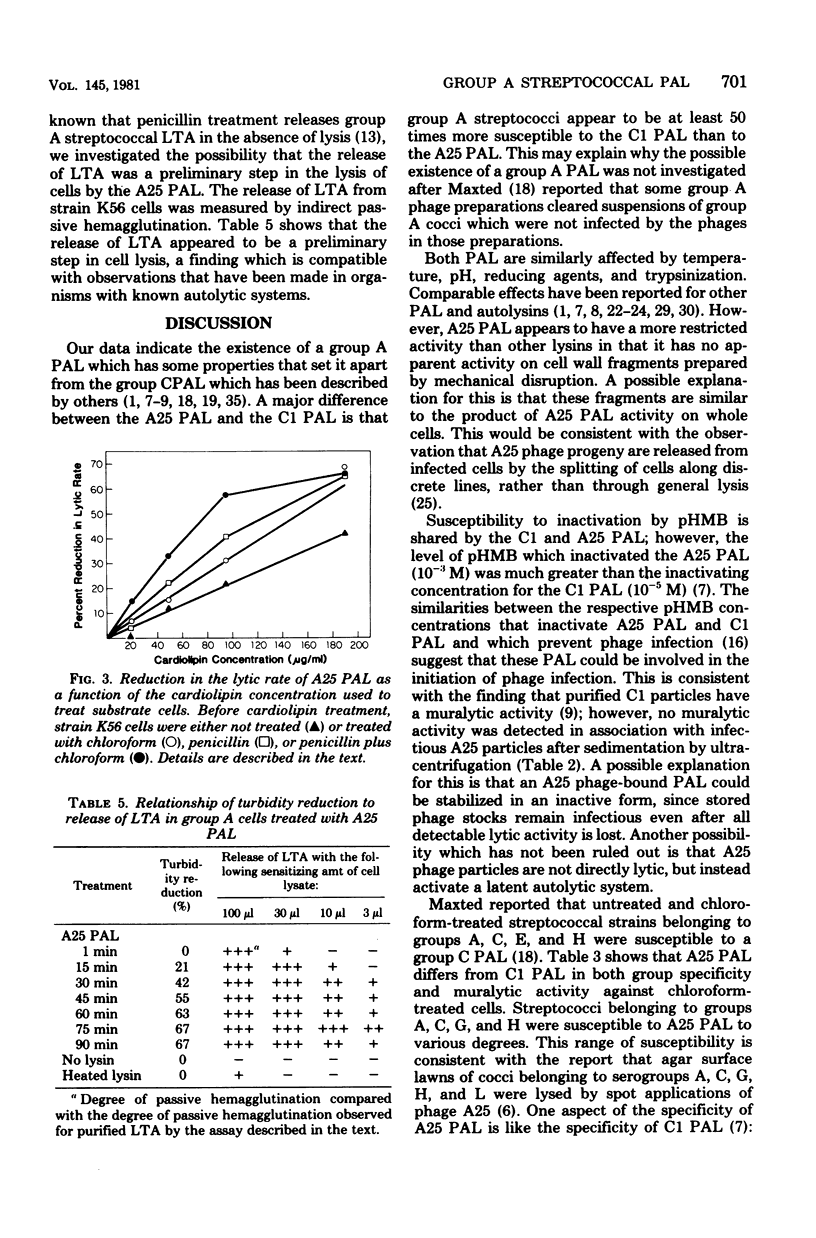
A phage-associated lysin was found in culture lysates resulting from the propagation of virulent bacteriophage A25 on the group A streptococcal strain designated K56. In contrast to the previously described group C streptococcal phage-associated lysins, A25 phage-associated lysin was more active on chloroform-treated cells, was not phage bound, and was active on some group G and H strains, as well as on group A and C strains. A25 phage-associated lysin had an optimum pH of 6.7 and was inactivated by 10(-3) M p-hydroxymercuribenzoate. Group A cells exposed to penicillin were more susceptible to A25 phage-associated lysin, whereas chloramphenicol-treated cells became resistant to lysis. Release of lipoteichoic acid appeared to precede lysis, and cardiolipin treatment of cells reversed the effects of chloroform and penicillin treatments. These results suggest the possibility that A25 phage-associated lysin may have a mechanism similar to the mechanism of an autolysin or that cell lysis may be due to the activation of an autolysin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKULIS S. S., SMITH C., BOLTRALIK J. J., HEYMANN H. STRUCTURE OF STREPTOCOCCAL CELL WALLS. IV. PURIFICATION AND PROPERTIES OF STREPTOCOCCAL PHAGE MURALYSIN. J Biol Chem. 1964 Dec;239:4027–4033. [PubMed] [Google Scholar]
- Cleary P. P., Wannamaker L. W., Fisher M., Laible N. Studies of the receptor for phage A25 in group A streptococci: the role of peptidoglycan in reversible adsorption. J Exp Med. 1977 Mar 1;145(3):578–593. doi: 10.1084/jem.145.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón A. E., Cole R. M., Leonard C. G. Intergroup lysis and transduction by streptococcal bacteriophages. J Virol. 1972 Mar;9(3):551–553. doi: 10.1128/jvi.9.3.551-553.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGHTY C. C., HAYASHI J. A. Enzymatic properties of a phage-induced lysin affecting group A streptococci. J Bacteriol. 1962 May;83:1058–1068. doi: 10.1128/jb.83.5.1058-1068.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX E. N., WITTNER M. K. OBSERVATIONS ON THE GROUP C STREPTOCOCCAL BACTERIOPHAGE AND LYTIC ENZYME SYSTEM. J Bacteriol. 1965 Feb;89:496–502. doi: 10.1128/jb.89.2.496-502.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Bernheimer A. W. Purification and physical properties of group C streptococcal phage-associated lysin. J Exp Med. 1971 May 1;133(5):1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L. Effect of macromolecular synthesis and lytic capacity on surface growth of Streptococcus faecalis. J Bacteriol. 1980 Feb;141(2):938–945. doi: 10.1128/jb.141.2.938-945.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks R. P., Daneo-Moore L., Shockman G. D. Relationship between cellular autolytic activity, peptidoglycan synthesis, septation, and the cell cycle in synchronized populations of Streptococcus faecium. J Bacteriol. 1978 Jun;134(3):1074–1080. doi: 10.1128/jb.134.3.1074-1080.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979 Mar;137(3):1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER D., KRAUSE R. M. INACTIVATION OF STREPTOCOCCAL BACTERIOPHAGE BY SULFHYDRYL REAGENTS. Proc Soc Exp Biol Med. 1963 Dec;114:822–826. doi: 10.3181/00379727-114-28810. [DOI] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAXTED W. R. The active agent in nascent phage lysis of streptococci. J Gen Microbiol. 1957 Jun;16(3):584–595. doi: 10.1099/00221287-16-3-584. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. Separation and properties of a red cell sensitizing substance from streptococci. J Bacteriol. 1966 Jun;91(6):2200–2204. doi: 10.1128/jb.91.6.2200-2204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M. Autolytic activity and its association with the development of competence in group H streptococci. J Bacteriol. 1973 Aug;115(2):607–614. doi: 10.1128/jb.115.2.607-614.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M., Cole R. M. Lysis of streptococci by an extracellular lysin produced by competent group H streptococcus strain CHALLIS. J Gen Microbiol. 1972 Jun;71(1):199–202. doi: 10.1099/00221287-71-1-199. [DOI] [PubMed] [Google Scholar]
- Ranhand J. M., Leonard C. G., Cole R. M. Autolytic activity associated with competent group H streptococci. J Bacteriol. 1971 Apr;106(1):257–268. doi: 10.1128/jb.106.1.257-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. E., Reed R. W. Electron microscopy of the replicative events of A25 bacteriophages in group A streptococci. Can J Microbiol. 1972 Jan;18(1):93–96. doi: 10.1139/m72-015. [DOI] [PubMed] [Google Scholar]
- Ronda-Lain C., Lopez R., Tapia A., Tomasz A. Role of the pneumococcal autolysin (murein hydrolase) in the release of progeny bacteriophage and in the bacteriophage-induced lysis of the host cells. J Virol. 1977 Jan;21(1):366–374. doi: 10.1128/jvi.21.1.366-374.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela M. N., Lahav M., Ginsburg I. Effect of leukocyte hydrolases on bacteria. IX. The release of lipoteichoic acid from group A streptococci and from Strep. mutans by leukocyte extracts and by lysozyme: relation to tissue damage in inflammatory sites. Inflammation. 1977 Jun;2(2):151–164. doi: 10.1007/BF00918677. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Cornett J. B., Mychajlonka M. Does penicillin kill bacteria?. Rev Infect Dis. 1979 Sep-Oct;1(5):787–796. doi: 10.1093/clinids/1.5.787. [DOI] [PubMed] [Google Scholar]
- Shungu D. L., Cornett J. B., Shockman G. D. Morphological and physiological study of autolytic-defective Streptococcus faecium strains. J Bacteriol. 1979 May;138(2):598–608. doi: 10.1128/jb.138.2.598-608.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonstein S. A., Hammel J. M., Bondi A. Staphylococcal bacteriophage-associated lysin: a lytic agent active against Staphylococcus aureus. J Bacteriol. 1971 Aug;107(2):499–504. doi: 10.1128/jb.107.2.499-504.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. From penicillin-binding proteins to the lysis and death of bacteria: a 1979 view. Rev Infect Dis. 1979 May-Jun;1(3):434–467. doi: 10.1093/clinids/1.3.434. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamaker L. W., Almquist S., Skjold S. Intergroup phage reactions and transduction between group C and group A streptococci. J Exp Med. 1973 Jun 1;137(6):1338–1353. doi: 10.1084/jem.137.6.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]


