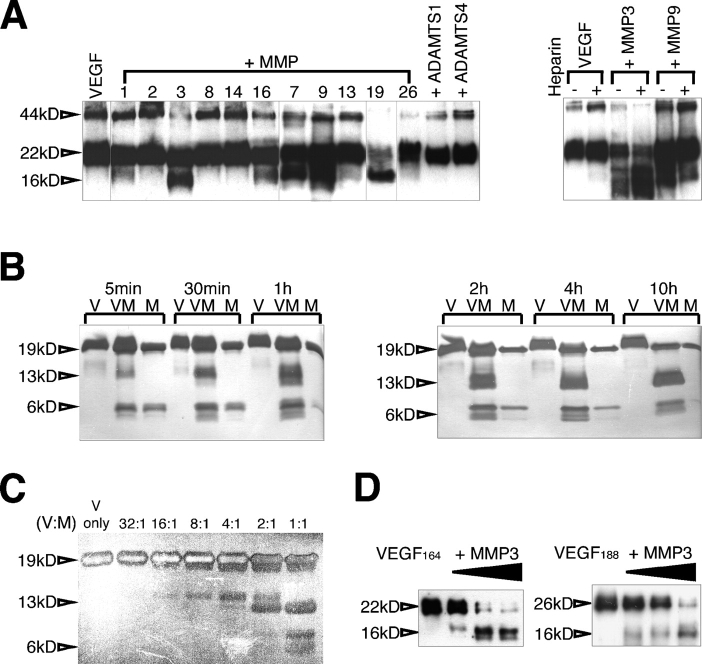
Figure 1.
VEGF-A is cleaved by a subset of MMPs. (A) Biotinylated mVEGF164 was incubated with the indicated MMPs. The digestion products were resolved in tricine gels and were detected by avidin-HRP. (right) The presence of heparin was tested to evaluate its effect in VEGF processing by MMP3 and MMP9. (B and C) mVEGF164 was incubated with MMP3 at different time points (B) and molar ratios (C) as indicated. VEGF cleavage was visualized by SDS-PAGE followed by silver staining. In these experiments, nonglycosylated mVEGF164 was used, hence the smaller size. (D) Both mVEGF164 and mVEGF188 were incubated with MMP3. VEGF cleavage was examined with immunoblots probed with an amino-terminal antibody (463; see Fig. 3 A). Glycosylated mVEGF164 was used for A and D, and nonglycosylated mVEGF164 was used for B and C. Note the faster mobility in B and C. 44 kD, glycosylated mVEGF164 dimer; 22 kD, glycosylated mVEGF164 monomer; 19 kD, nonglycosylated mVEGF164 monomer; 16 kD, glycosylated mVEGF164-cleaved monomer fragment; 13 kD, nonglycosylated mVEGF164-cleaved monomer fragment; 6 kD, mVEGF164-cleaved monomer fragment. V, VEGF; M, MMP3; VM, VEGF + MMP3; ADAMTS, a disintegrin and metalloproteinase domain with thrombospondin repeats.