Abstract
The myosin V carboxyl-terminal globular tail domain is essential for the attachment of myosin V to all known cargoes. Previously, the globular tail was viewed as a single, functional entity. Here, we show that the globular tail of the yeast myosin Va homologue, Myo2p, contains two structural subdomains that have distinct functions, namely, vacuole-specific and secretory vesicle–specific movement. Biochemical and genetic analyses demonstrate that subdomain I tightly associates with subdomain II, and that the interaction does not require additional proteins. Importantly, although neither subdomain alone is functional, simultaneous expression of the separate subdomains produces a functional complex in vivo. Our results suggest a model whereby intramolecular interactions between the globular tail subdomains help to coordinate the transport of multiple distinct cargoes by myosin V.
Introduction
Organelles of eukaryotic cells are accurately partitioned during cell division. The proper distribution of organelles between mother and daughter cells generally requires motor proteins that bind to the organelles and move them along microtubules and/or actin filaments. The same molecular motors are crucial for the polarized movement of vesicles in differentiated cells. In most eukaryotes, microtubules mediate long-range transport of membranous organelles from the cell center to the periphery and from the cell surface to the cell center, whereas actin mediates short-range transport and anchorage at the cell periphery. In contrast, in the yeast Saccharomyces cerevisiae, most intracellular compartments move solely on actin.
Myosin V motors (in plants, myosin XI) function in actin-based movement and are present in most eukaryotes. Some myosin V motors transport multiple cargoes. For example, vertebrate myosin Va moves melanosomes in melanocytes (Provance et al., 1996; Nascimento et al., 1997; Wu et al., 1997; Rogers and Gelfand, 1998), the smooth ER in brain Purkinje cells (Takagishi et al., 1996), membranous vesicles in nerve cells (Evans et al., 1998), and chromaffin vesicles (Rose et al., 2003). Moreover, within a single cell type, myosin Va likely moves more than one type of cargo (da Silva Bizario et al., 2002). In S. cerevisiae, the myosin V, Myo2p, is essential and transports secretory vesicles, the vacuole, late Golgi, and peroxisomes (Govindan et al., 1995; Hill et al., 1996; Catlett and Weisman, 1998; Schott et al., 1999; Karpova et al., 2000; Hoepfner et al., 2001; Rossanese et al., 2001). Myo2p is also required for orientation of the mitotic spindle (Beach et al., 2000; Yin et al., 2000) and is involved in mitochondrial inheritance (Boldogh et al., 2004; Itoh et al., 2004).
Myosin V contains two identical heavy chains, organized into four domains (Cheney et al., 1993; see Fig. 1 A). The amino-terminal motor domain contains binding sites for both actin and ATP. The neck contains six IQ motifs that bind calmodulin and other regulatory light chains. The α-helical coiled-coil region is required for dimerization of the heavy chains. The fourth domain, the globular tail, binds to cargoes through interaction with organelle-specific receptors.
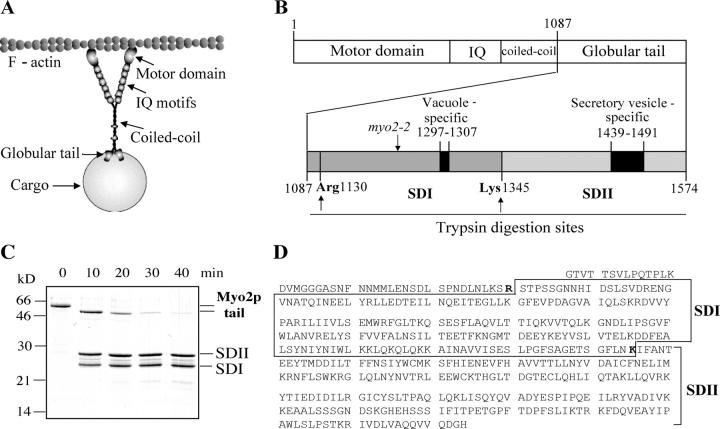
Figure 1.
The Myo2p globular tail has two subdomains. (A) Schematic of domains of Myo2p based on chick brain myosin V (Cheney et al., 1993), adapted from Cope et al. (1996). (B) Domains of Myo2p with an expanded region of the globular tail indicating subdomains I and II. Vacuole-specific point mutants reside within subdomain I; most secretory vesicle–specific mutants reside within subdomain II. (C) Trypsin digestion of the globular tail results in two stable polypeptides, subdomain I (SDI) and subdomain II (SDII). Globular tail was incubated with trypsin at 0°C. Samples were collected at 10-min intervals and analyzed by SDS-PAGE (15%). Proteins were detected with Coomassie blue R-250. (D) Amino acid sequence of the Myo2p globular tail (residues 1087–1574). Exposed trypsin cleavage sites in bold.
Organelle-specific myosin V receptors play critical roles in the regulation of cargo transport. Indeed, the vacuole-specific Myo2p receptor is required for both the temporal and the spatial regulation of vacuole movement. At a specific point in the cell cycle, Myo2p attaches to the vacuole via the Vac17p–Vac8p receptor complex and moves the vacuole to the bud. Once the vacuole arrives in the bud, Vac17p is degraded, depositing the vacuole in its correct location (Tang et al., 2003).
Point mutations in Myo2p that specifically affect vacuole movement reveal a region of the Myo2p globular tail that binds to the vacuole-specific Myo2p receptor, Vac17p. This region is distinct from a second area specifically required for secretory vesicle movement. Point mutations that affect vacuole movement are in the proximal half of the globular tail (Catlett and Weisman, 1998; Catlett et al., 2000), whereas most of the mutations that affect secretion but not vacuole movement are in the distal half (Schott et al., 1999; Catlett et al., 2000). For example, although yeast with full-length Myo2p-ΔAflII as the sole copy of Myo2p are not viable, the Myo2p-ΔAflII allele supports vacuole movement (Catlett et al., 2000). Thus, there are at least two functionally distinct regions of the Myo2p globular tail: one for vacuole movement and the other for secretory vesicle movement.
Here, we show that the Myo2p globular tail contains two structural subdomains that correspond to two functional regions. Although a region within subdomain I is specific for vacuoles and a region within subdomain II is specific for secretory vesicles, neither subdomain functions on its own; their association is needed for each Myo2p function. This organization may allow the Myo2p globular tail to play an active role in specifying cargo.
Results and discussion
Identification of subdomains I and II of the Myo2p tail
To examine the structural basis of Myo2p–cargo interactions, we generated a bacterially expressed GST fusion protein of the tail of Myo2p (residues 1087–1574). Removal of the GST tag by digestion at an engineered thrombin cleavage site yielded a polypeptide with the expected size of ∼55 kD (Fig. 1 C and Table SI [available at http://www.jcb.org/cgi/content/full/jcb.200407146/DC1]). To map the structure of the tail, we performed mild proteolysis. Trypsin digestion of the Myo2p tail at 0°C resulted in three major polypeptides of ∼50, 26, and 24 kD (Fig. 1 C). Based on the amino acid sequence and the sizes of these fragments, we concluded that trypsin cleaved after Arg1130 and Lys1345 (Fig. 1 D). Arg1130 was the most sensitive to proteolysis and was responsible for the 50-kD fragment (residues 1131–1574). Notably, the same polypeptide, when overexpressed as a GST fusion, completely blocked yeast growth (Schott et al., 1999). Based on these findings, we define the functional globular tail as residues 1131–1574.
This 50-kD fragment was cleaved by trypsin into two polypeptides (Fig. 1) that correspond to residues 1131–1345 (24 kD) and residues 1346–1574 (26 kD) (Table SI). These polypeptides will be referred to as subdomain I and subdomain II, respectively. The predicted 6-kD fragment of residues 1087–1130 was not detected by mass spectrometry.
Notably, all seven vacuole-specific myo2 point mutants reside within subdomain I (Catlett et al., 2000; Fig. 1 B). Similarly, most secretory vesicle–specific mutants map to subdomain II (Schott et al., 1999; Catlett et al., 2000; Fig. 1 B). These observations suggest that subdomains I and II bind distinct cargoes.
Subdomain I interacts with subdomain II
In a yeast two-hybrid screen designed to identify vacuole-specific Myo2p binding proteins using subdomain I as the bait (Myo2p residues 1139–1345), a clone containing subdomain II (Myo2p residues 1336–1574) was obtained (Catlett, 2000). This finding strongly suggested that subdomain I interacts with subdomain II. To test this further, subdomain II (residues 1346–1574) was subcloned into a yeast two-hybrid vector. Subdomain II strongly interacted with subdomain I (Fig. 2 A).
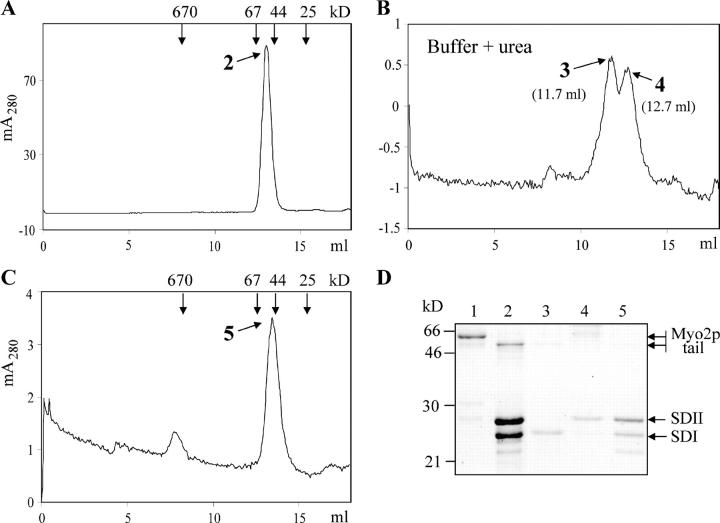
Figure 2.
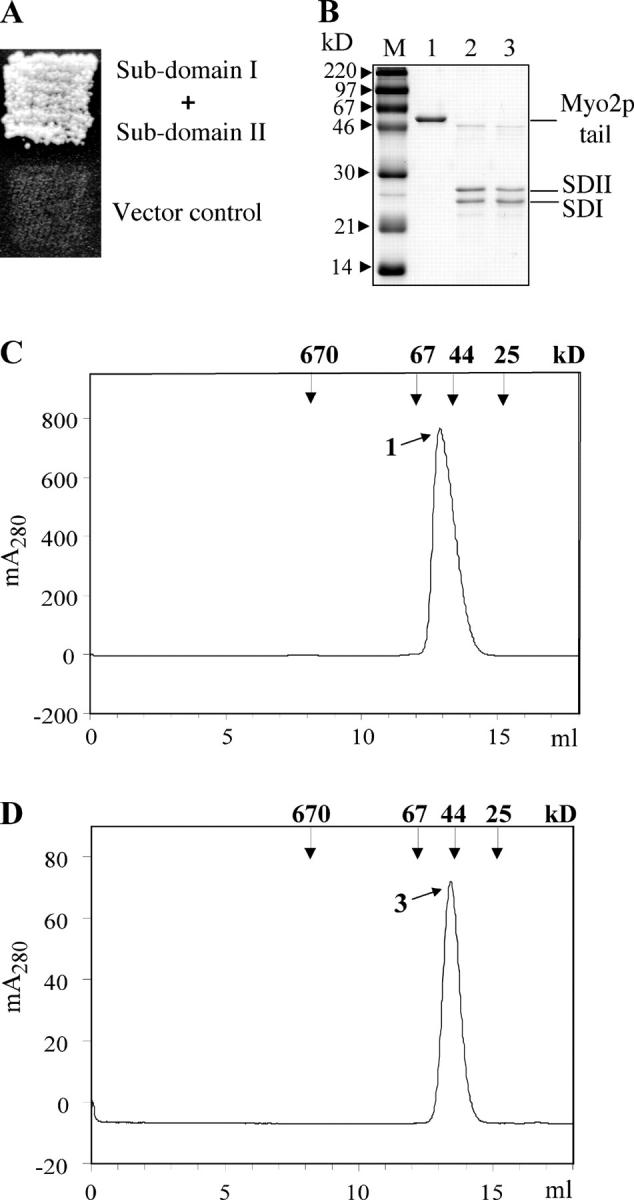
Subdomain I interacts with subdomain II. (A) In a yeast two-hybrid test, subdomain I (residues 1139–1345) fused with GAL4 BD interacts with subdomain II (residues 1346–1574) fused with GAL4 AD. (B) Purified Myo2p tail (lane 1 and C, peak 1) digested with trypsin (lane 2), collected after gel filtration (lane 3 and D, peak 3), and analyzed by SDS-PAGE. M, molecular mass markers. (C) Purified Myo2p tail analyzed by gel filtration on Superose 12. (D) The subdomains coeluted as a single peak at an elution position of ∼50 kD. (C and D) Vertical arrows indicate protein standards (Bio-Rad Laboratories).
Interaction of subdomains I and II may occur within a single polypeptide. The interaction could be intrinsic to the structure of the Myo2p globular tail or, alternatively, additional yeast proteins may mediate the interaction. In a yeast two-hybrid test, other yeast proteins are available that potentially can enhance or inhibit an interaction. Therefore, we used a bacterially expressed recombinant Myo2p tail encoding residues 1087–1574 to examine the association of subdomains I and II in vitro.
Subdomains I and II generated by mild proteolysis of the Myo2p tail were analyzed by gel filtration. The resultant polypeptides coeluted as a single peak (Fig. 2 D) at an elution position similar to that of the full-length tail (Fig. 2 C). Denaturing SDS-PAGE demonstrated that over 90% of the protein had been cleaved into two polypeptides of 24 and 26 kD (Fig. 2 B, lane 2).
When subdomains I and II were incubated in 6 M urea and analyzed by gel filtration with a buffer containing 6 M urea, two partially resolved peaks were observed (Fig. 3 B). When the urea was removed by spin filtration, the two polypeptides rapidly reassociated, as is evidenced by their coelution at the position of a 50-kD monomer upon gel filtration without urea (Fig. 3, A and C). These findings suggest a tight intrinsic association of subdomains I and II. Indeed, size exclusion chromatographic simulations of the reversible dissociation of the 50-kD protein into its 24- and 26-kD components were obtained using an extension of a program described previously (Shalongo et al., 1993). Simulations of the profiles observed after injection of aliquots of the 50-kD protein, at either 0.03 or 0.1 mg/ml, into Superose 12 require that the dissociation equilibrium constant be ≤1 nM.
Figure 3.
Subdomains I and II partially separate in the presence of 6 M urea and reassociate upon removal of urea. (A) Globular tail (D, lane 1) digested with trypsin for 20 min at 0°C and subjected to gel filtration (peak 2 and D, lane 2). Subdomains coeluted at ∼50 kD. Peak 2 incubated in the presence of 6 M urea for 2 h on ice. Vertical arrows indicate protein standards. (B) Half the sample loaded on Superose 12 and equilibrated with Hepes buffer containing 6 M urea. Subdomain I (peak 3 and D, lane 3) and subdomain II (peak 4 and D, lane 4) partially separated. Urea was removed from the nonchromatographed sample with a spin filter; samples were immediately applied to a Superose12 column and equilibrated with Hepes buffer without urea. (C) Subdomains I and II coeluted at ∼50 kD (peak 5 and D, lane 5). Vertical arrows indicate protein standards. (D) Indicated fractions from A, B, and C analyzed by SDS-PAGE (15%).
Myo2p forms a dimer through its coiled-coil domain (Cheney et al., 1993); if the coiled-coil region is absent, the Myo2p globular tail does not interact with itself (Beningo et al., 2000). Although the globular tail from each heavy chain contains both subdomains, a tight intratail association of the subdomains likely prevents dimer formation. To test this model, we analyzed the behavior of the globular tail in solution by equilibrium analytical ultracentrifugation. The globular tail behaved as a monomer of the predicted molecular mass of ∼55 kD (Fig. S1; available at http://www.jcb.org/cgi/content/full/jcb.200407146/DC1). Thus, the interaction between subdomains I and II occurs within a single polypeptide.
Subdomain I and II interactions are required for Myo2p tail function
Although subdomains I and II appear to bind distinct cargoes, neither subdomain I nor subdomain II functions independently in vacuole or secretory vesicle movement. Overexpression of the globular tail of Myo2p is lethal (Reck-Peterson et al., 1999; Schott et al., 1999; Catlett et al., 2000), and overexpression of the globular tail missing the secretory vesicle–binding domain, Myo2p-ΔAflII, causes a vacuole inheritance defect (Catlett et al., 2000). In contrast, overexpression of a truncated globular tail (residues 1111–1430; not depicted) or of the individual subdomains I or II (wild type or ΔAflII) did not disrupt growth or vacuole inheritance (Fig. 4). These results suggest that neither subdomain can function independently. However, the simultaneous overexpression of subdomains I and II (wild type or ΔAflII) as separate polypeptides caused a vacuole inheritance defect similar to that caused by overexpression of the full-length globular tail (Fig. 4 A). This result was obtained even though the protein expression levels of subdomains I and II were approximately threefold less than those of the full-length globular tail (Fig. 4 C).
Figure 4.
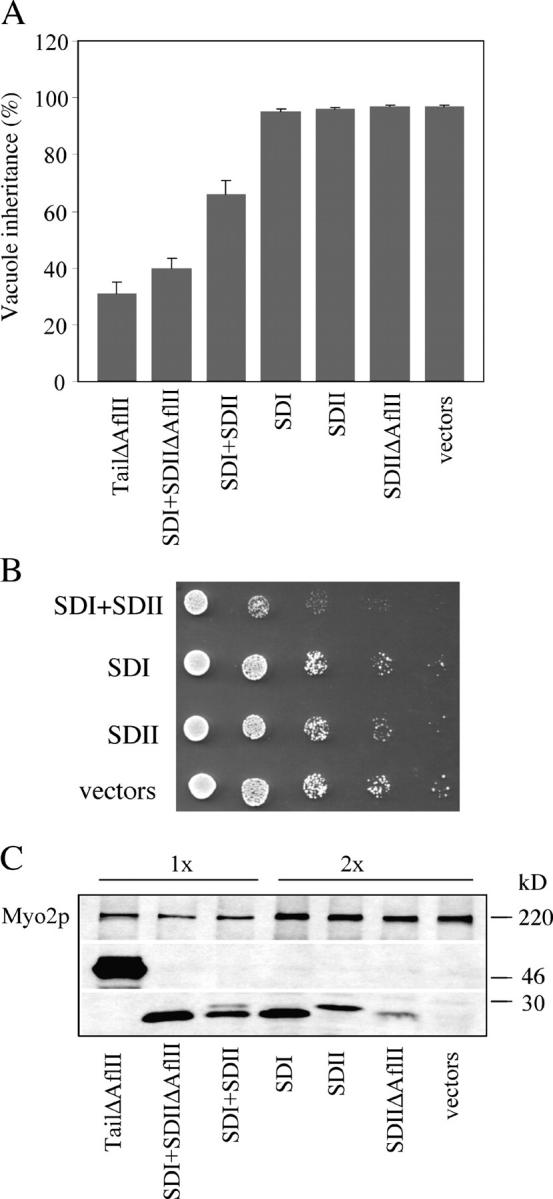
Coexpression of subdomains I and II has dominant negative effects. (A) Coexpression of subdomains I and II blocks vacuole inheritance. LWY7235 cells with the following plasmid pairs: Tail-ΔAflII, pMYO2 tail-ΔAflII (Δ1459–1491); vector; SDI + SDII-ΔAflII, pSubdomain I, pSubdomain II-ΔAflII; SDI + SDII, pSubdomain I, pSubdomain II; SDI, pSubdomain I, vector; SDII, pSubdomain II, vector; SDII-ΔAflII, pSubdomain II-ΔAflII, vector; vectors, vectors only. Strains were grown in SC-His-Ura media at 24°C. Vacuoles were visualized with FM4-64. Data are presented as a percentage of budded cells with inherited vacuoles and correspond to the mean and SD of samples from three independent transformations, with a minimum total of 200 cells scored. (B) Coexpression of subdomains I and II inhibited cell growth. Fivefold serial dilutions were applied to SC-His-Ura plates, incubated at 37°C, and photographed after 2 d. Growth of at least three independent transformants was assessed. Results shown are representative of independent transformants. (C) Protein expression was assessed by Western blot (anti-Myo2p tail antiserum). Cell extracts were applied to SDS-PAGE (4–15% gradient). Twice as much cell extract was applied in the lanes labeled “2x.”
Simultaneous overexpression of wild-type subdomains I and II decreased yeast growth at 37°C (Fig. 4 B). Secretory vesicles are the only identified essential cargo of Myo2p. Thus, overexpression of subdomain I with subdomain II missing the secretory vesicle–specific region (ΔAflII) did not affect growth (unpublished data). These results strongly suggest that although neither subdomain I nor subdomain II can function independently, when coexpressed in vivo, subdomains I and II associate, and the complex executes the functions of the intact globular tail.
Myo2p attaches to the vacuole through a direct interaction with Vac17p (Catlett, 2000; Ishikawa et al., 2003). Therefore, we tested whether subdomain I alone interacts with Vac17p. Although subdomain I contains the vacuole-specific receptor binding region, it did not interact with Vac17p. Similarly, subdomain II did not interact with Vac17p. However, when subdomains I and II were expressed simultaneously as separate polypeptides, an interaction with Vac17p was observed (Fig. 5 A). Thus, the separate subdomain I and II polypeptides can function in vivo, but only if they form a complex with each other.
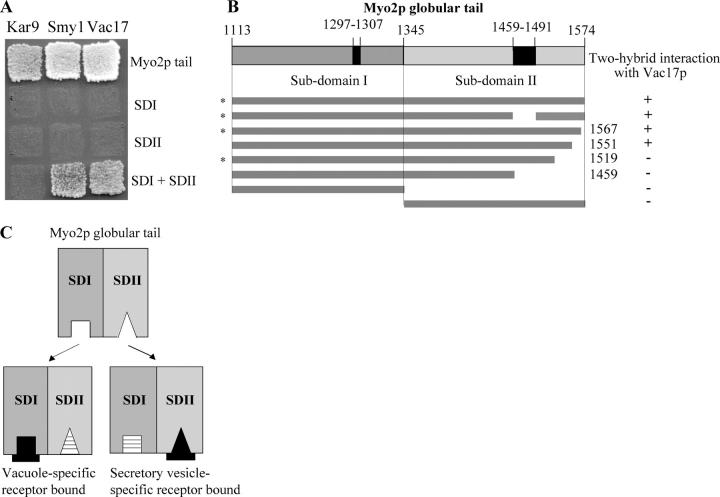
Figure 5.
Subdomains I and II coexpressed as separate polypeptides interact with Vac17p and Smy1p. (A) The Myo2p tail interacts with Vac17p, Smy1p, and Kar9p in a yeast two-hybrid test (top). Neither subdomain I nor II alone interacts with these proteins (middle rows). Simultaneous expression of subdomain I with subdomain II resulted in an interaction with Vac17p and Smy1p (bottom). Strains contained three plasmids (protein fusions and/or vectors). Plates were photographed on day 4. (B) The carboxyl terminus of the Myo2p globular tail is required for Myo2p–Vac17p interactions. Plasmids encoding GAL4 BD fused with the indicated regions of the MYO2 globular tail were tested for interaction with GAL4 AD fused with VAC17-encoding residues 1–170. Expression of noninteracting constructs was confirmed by Western blot analysis (not depicted). Asterisks indicate constructs reported by Ishikawa and colleagues (2003). (C) A model showing that the association of subdomains I and II may coordinate vacuole and secretory vesicle movement. Occupancy of the vacuole-specific receptor binding site (square) in subdomain I may cause a conformational change in subdomain II that precludes binding of the putative secretory vesicle receptor complex (triangle). Similarly, binding of the secretory vesicle receptor may prevent the binding of the vacuole-specific receptor.
Myo2p tails containing the deletions Δ1459–1574 or Δ1519–1574 did not interact with Vac17p, whereas neither a small deletion within the subdomain II secretory vesicle–specific region (Δ1459–1491) nor truncations of the carboxyl-terminal residues 1567–1574 and 1551–1574 interfered with Myo2p–Vac17p association (Fig. 5 B). These results suggest that a region of subdomain II between residues 1519 and 1551 is important for association with subdomain I and enables subdomain I to bind Vac17p. Alternatively, a region within subdomain II may join with a region of subdomain I to form the vacuole-binding region.
Similar to results obtained from wild-type treatment, mild proteolysis of a GST fusion protein of the truncated globular tail (1131–1567) resulted in two stable polypeptides of ∼24 and 26 kD, which tightly associated and comigrated on gel filtration (unpublished data). Likewise, this truncated globular tail interacts with Vac17p in a yeast two-hybrid test (Fig. 5). Unfortunately, biochemical analysis of further truncations was not feasible. Neither 1131–1551 nor 1131–1519 could be expressed as stable polypeptides.
In addition to Vac17p, Kar9p and Smy1p also bind directly to the Myo2p globular tail (Beach et al., 2000; Beningo et al., 2000; Yin et al., 2000). Kar9p is required for mitotic spindle orientation in budding yeast (Miller and Rose, 1998). SMY1 was identified as a multicopy suppressor of the myo2-66 mutant (Lillie and Brown, 1992) and several other myo2ts mutants (Schott et al., 1999); the function of Smy1p is unknown. The specific regions on the Myo2p tail that bind to either Kar9p or Smy1p have not yet been determined. Neither Kar9p nor Smy1p interacts with either subdomain I or subdomain II alone (Fig. 5 A).
Like Vac17p, Smy1p interacts with subdomain I or II when the subdomains are coexpressed (Fig. 5 A). Thus, association between subdomains I and II is important for Myo2p binding to many cargoes. The simultaneous expression of subdomains I and II did not reveal an interaction with Kar9p. However, this may have been caused by technical limits of the assay. For Vac17p and Smy1p, we used regions that interact strongly with Myo2p (i.e., Vac17p-(1–170) and Smy1p-(465–656)). For Kar9p, a similar region has not been identified. Note that the Myo2p tail interacts more strongly with the truncated proteins than with the corresponding full-length proteins of Vac17p or Smy1p (Beningo et al., 2000; Catlett, 2000; Ishikawa et al., 2003).
Association of subdomains I and II may specify the attachment of cargoes
Two features of the globular tail may be important for cargo attachment. The first feature is a binding site for a cargo-specific receptor. The second feature is composed of the structural determinants that mediate the intramolecular association of subdomains I and II. This interaction may promote the proper conformation of the specific receptor-binding site in each subdomain. We cannot yet determine whether subdomains I and II require each other for function and/or for folding. However, myo2-20, which has two point mutations in subdomain I, is partially defective in secretory vesicle movement, even though the secretory vesicle–binding region is in subdomain II (Schott et al., 1999). This mutant supports the hypothesis that subdomain I and II interactions are important for function. Unfortunately, the myo2-20 tail protein was not stable and could not be analyzed further.
It is tempting to speculate that interaction of the two subdomains may coordinate myosin V attachment to distinct cargoes. Binding of the secretory vesicle receptor to subdomain II may induce conformational changes in subdomain I and thereby close the binding site for other types of cargo; i.e., the vacuole (Fig. 5 C). Similarly, binding of the vacuole-specific receptor (or of another cargo-specific receptor) to subdomain I may result in conformational changes that prevent binding of the secretory vesicle–specific receptor to subdomain II. A high resolution structure of the globular tail is needed for a more definitive view of the organization of the globular tail.
One caveat of the model is that although the globular tails are monomers, myosin V is a dimer. Therefore, there may be communication between the dimerization domains as well. A second caveat is that if myosin V binds to a receptor complex that is already attached to the membrane, then steric hindrance could suffice to preclude binding of more than one type of cargo. However, a small fraction of yeast Myo2 is cytosolic (Reck-Peterson et al., 1999), which raises the possibility that Myo2 may initially bind its adaptor proteins in the cytoplasm.
The two subdomains within the Myo2p tail are conserved among the myosin V motors. Based on the alignment of Myo2p and myosin Va from mouse, rat, and chicken, we propose regions that correspond to subdomains I and II (Supplemental text and Fig. S2; available at http://www.jcb.org/cgi/content/full/jcb.200407146/DC1).
Vertebrate myosin Va has a second region that plays a key role in binding to cargo. Upstream of the globular tail of myosin Va is a medial tail domain that varies depending on alternative splicing. The resulting six myosin Va isoforms play unique roles in specifying cargo. Exon F mediates the interaction of the myosin Va globular tail with melanosomes, whereas isoforms lacking exon F but containing exon D are associated with vesicles near the Golgi (Au and Huang, 2002; Westbroek et al., 2003). Thus, myosin Va may use at least two mechanisms to regulate attachment to distinct cargoes: multiple isoforms in the medial tail generate cargo-specific domains and, as with yeast Myo2p, specificity may be regulated by subdomain I and subdomain II interactions within the globular tail.
Materials and methods
Expression and purification of the recombinant Myo2p globular tail
The GST-Myo2p tail fusion protein was expressed in Escherichia coli from pGM2G. Cells were grown in Luria broth media with 0.1 mg/ml ampicillin at 37°C to an OD600 of ∼0.6. After 4 h of IPTG-induced expression at 30°C, cells were harvested and frozen at −80°C. Thawed cells (from 1 liter of media) were resuspended in 20 ml Hepes lysis buffer (20 mM Hepes, pH 7.5, 200 mM NaCl, and 1 mM EDTA) with one tablet of protease inhibitors cocktail EDTA free (Roche), and then subjected to French press disruption at 1,000 psi. The cell lysate was centrifuged at 27,000 g for 30 min. Recombinant GST fusion proteins were purified on a glutathione Sepharose 4B resin (Amersham Biosciences) using Hepes lysis buffer. After incubation, the resin was resuspended in Hepes lysis buffer, applied to a small disposable column, and washed until the flow-through had an absorbance OD280 of ∼0. The GST tag was removed by proteolytic cleavage with biotinylated thrombin (Novagen) as follows. The resin with bound protein was equilibrated with thrombin cleavage buffer and incubated with five units of thrombin per 1 ml of the buffer for 3 h at 20°C, with subsequent removal of the protease on streptavidin agarose beads. Cleaved proteins were dialyzed overnight against column buffer (20 mM Hepes buffer, pH 6.5, 0.1 M NaCl, and 1 mM EDTA) at 4°C, concentrated by spin filtration with Ultrafree-4 centrifugal filter 30-K membrane (Millipore), and purified by gel filtration on a prep grade column (HiLoad 16/60 Superdex 200; Amersham Biosciences).
Protein analysis
The amino-terminal sequences of the expressed protein and the fragments that resulted from trypsin cleavage were determined using a Procise 492 Sequencer (Applied Biosystems) after the proteins were electroblotted onto ProBlott membrane. Protein samples were further analyzed using matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry by the University of Iowa facilities. Before analysis, the purified samples were concentrated-desalted with a ZipTipC4 pipette tip (Millipore) containing reverse-phase media, according to the vendor's instructions.
For mild proteolysis, purified Myo2p tail was concentrated to ∼0.5 mg/ml and incubated at 0°C with 3 μg/ml of trypsin (Sigma-Aldrich) for 10–40 min. To stop the reaction, 0.1 mg/ml of Pefabloc SC (Roche) was added and the mixture was incubated for 10 min on ice before being analyzed by SDS-PAGE or gel filtration on a Superose 12 HR 10/30 column (Amersham Biosciences).
Levels of yeast protein expression were assessed by Western blot analysis. Cell extracts were separated on SDS-PAGE (10% or 4–15% gel) and transferred to nitrocellulose membranes overnight. The membranes were probed with goat anti–Myo2p tail antiserum at 1:2,000 dilution (Catlett et al., 2000) or rabbit anti–Gal4 DNA activation domain (GAL4 AD) antiserum at 1:2,000 dilution (Upstate Biotechnology).
Yeast two-hybrid assay
The yeast strain PJ69-4A was cotransformed with the indicated GAL4 AD fusion construct (LEU2 marker) and the indicated Gal4 DNA-binding domain (GAL4 BD) fusion constructs (TRP1 marker; James et al., 1996). Single colonies of each transformant were patched onto SC-Leu-Trp plates. After growth for 2 d at 24°C, the patches were replica plated to test plates; SC-Leu-Trp-His-Ade media contained 30 mM 3-amino-triazole (Sigma-Aldrich). Plates were incubated for 4–5 d at 24°C.
For the three-hybrid assay, an additional GAL4 BD fusion construct in a plasmid with a URA3 marker was used. DNA sequences encoding Vac17p (residues 1–170), Smy1p (residues 465–656), and full-length Kar9p were fused with GAL4 AD and transformed into yeast strain PJ69-4A together with the full-length Myo2p tail (GAL4 BD fusion; TRP1 marker), Myo2p tail subdomain I (GAL4 BD fusion; TRP1 marker), and/or subdomain II (GAL4 BD fusion; URA3 marker).
Online supplemental material
The supplemental Materials and methods section describes plasmids construction, analytical ultracentrifugation, and vacuole visualization. Online supplemental Results section provides a comparative sequence analysis of yeast myosin Va from mouse, rat, and chicken. The analysis suggests that subdomains I and II are a common feature of myosin V motors. Table SI contains MALDI-TOF mass spectrometry data of peptides generated via mild trypsinolysis of the Myo2p tail. Fig. S1 presents the equilibrium analytical ultracentrifugation studies. Fig. S2 presents the alignment of the myosin Va globular tail from mouse, rat, chicken, and S. cerevisiae. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200407146/DC1.
Acknowledgments
We thank Dr. E. Stellwagen for simulations of a dissociation equilibrium constant for subdomains I and II, Dr. S. Ramaswamy for helpful discussions and advice, J. Duex and other members of L.S. Weisman's laboratory for a critical reading of this manuscript, and Dr. L.M. Teesch and Y. Li for MALDI-TOF mass spectrometry.
This work was supported by National Institutes of Health grant GM62261 and an Established Investigator Award from the American Heart Association (to L.S. Weisman). N.L. Catlett was supported by National Institute on Aging grant T32 AG 00214 to the Interdisciplinary Research Training Program on Aging at the University of Iowa.
N.L. Catlett's present address is 737 Snapdragon St., Encinitas, CA 92024.
Abbreviations used in this paper: GAL4 AD, Gal4 DNA activation domain; GAL4 BD, Gal4 DNA-binding domain; MALDI-TOF, matrix-assisted laser desorption/ionization time of flight.
References
- Au, J.S., and J.D. Huang. 2002. A tissue-specific exon of myosin Va is responsible for selective cargo binding in melanocytes. Cell Motil. Cytoskeleton. 53:89–102. [DOI] [PubMed] [Google Scholar]
- Beach, D.L., J. Thibodeaux, P. Maddox, E. Yeh, and K. Bloom. 2000. The role of the proteins Kar9 and Myo2 in orienting the mitotic spindle of budding yeast. Curr. Biol. 10:1497–1506. [DOI] [PubMed] [Google Scholar]
- Beningo, K.A., S.H. Lillie, and S.S. Brown. 2000. The yeast kinesin-related protein Smy1p exerts its effects on the class V myosin Myo2p via a physical interaction. Mol. Biol. Cell. 11:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh, I.R., S.L. Ramcharan, H.C. Yang, and L.A. Pon. 2004. A type V myosin (Myo2p) and a Rab-like G-protein (Ypt11p) are required for retention of newly inherited mitochondria in yeast cells during cell division. Mol. Biol. Cell. 15:3994–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett, N.L. 2000. Role of a yeast myosin-V in movement of the vacuole and other cargoes. Ph.D. thesis. University of Iowa, Iowa City, IA. 135 pp.
- Catlett, N.L., and L.S. Weisman. 1998. The terminal tail region of a yeast myosin-V mediates its attachment to vacuole membranes and sites of polarized growth. Proc. Natl. Acad. Sci. USA. 95:14799–14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett, N.L., J.E. Duex, F. Tang, and L.S. Weisman. 2000. Two distinct regions in a yeast myosin-V tail domain are required for the movement of different cargoes. J. Cell Biol. 150:513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney, R.E., M.K. O'Shea, J.E. Heuser, M.V. Coelho, J.S. Wolenski, E.M. Espreafico, P. Forscher, R.E. Larson, and M.S. Mooseker. 1993. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 75:13–23. [DOI] [PubMed] [Google Scholar]
- Cope, M.J., J. Whisstock, I. Rayment, and J. Kendrick-Jones. 1996. Conservation within the myosin motor domain: implications for structure and function. Structure. 4:969–987. [DOI] [PubMed] [Google Scholar]
- da Silva Bizario, J.C., A.A. da Cunha Nascimento, L. Casaletti, E.V. Patussi, M.F. Chociay, R.E. Larson, and E.M. Espreafico. 2002. Expression of constructs of the neuronal isoform of myosin-Va interferes with the distribution of melanosomes and other vesicles in melanoma cells. Cell Motil. Cytoskeleton. 51:57–75. [DOI] [PubMed] [Google Scholar]
- Evans, L.L., A.J. Lee, P.C. Bridgman, and M.S. Mooseker. 1998. Vesicle-associated brain myosin-V can be activated to catalyze actin-based transport. J. Cell Sci. 111:2055–2066. [DOI] [PubMed] [Google Scholar]
- Govindan, B., R. Bowser, and P. Novick. 1995. The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 128:1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, K.L., N.L. Catlett, and L.S. Weisman. 1996. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J. Cell Biol. 135:1535–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner, D., M. van den Berg, P. Philippsen, H.F. Tabak, and E.H. Hettema. 2001. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J. Cell Biol. 155:979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, K., N.L. Catlett, J.L. Novak, F. Tang, J.J. Nau, and L.S. Weisman. 2003. Identification of an organelle-specific myosin V receptor. J. Cell Biol. 160:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, T., E.A. Toh, and Y. Matsui. 2004. Mmr1p is a mitochondrial factor for Myo2p-dependent inheritance of mitochondria in the budding yeast. EMBO J. 23:2520–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P., J. Halladay, and E.A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 144:1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova, T.S., S.L. Reck-Peterson, N.B. Elkind, M.S. Mooseker, P.J. Novick, and J.A. Cooper. 2000. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell. 11:1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie, S.H., and S.S. Brown. 1992. Suppression of a myosin defect by a kinesin-related gene. Nature. 356:358–361. [DOI] [PubMed] [Google Scholar]
- Miller, R.K., and M.D. Rose. 1998. Kar9p is a novel cortical protein required for cytoplasmic microtubule orientation in yeast. J. Cell Biol. 140:377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento, A.A., R.G. Amaral, J.C. Bizario, R.E. Larson, and E.M. Espreafico. 1997. Subcellular localization of myosin-V in the B16 melanoma cells, a wild-type cell line for the dilute gene. Mol. Biol. Cell. 8:1971–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provance, D.W., Jr., M. Wei, V. Ipe, and J.A. Mercer. 1996. Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution. Proc. Natl. Acad. Sci. USA. 93:14554–14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson, S.L., P.J. Novick, and M.S. Mooseker. 1999. The tail of a yeast class V myosin, myo2p, functions as a localization domain. Mol. Biol. Cell. 10:1001–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, S.L., and V.I. Gelfand. 1998. Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr. Biol. 8:161–164. [DOI] [PubMed] [Google Scholar]
- Rose, S.D., T. Lejen, L. Casaletti, R.E. Larson, T.D. Pene, and J.M. Trifaro. 2003. Myosins II and V in chromaffin cells: myosin V is a chromaffin vesicle molecular motor involved in secretion. J. Neurochem. 85:287–298. [DOI] [PubMed] [Google Scholar]
- Rossanese, O.W., C.A. Reinke, B.J. Bevis, A.T. Hammond, I.B. Sears, J. O'Connor, and B.S. Glick. 2001. A role for actin, Cdc1p, and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J. Cell Biol. 153:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott, D., J. Ho, D. Pruyne, and A. Bretscher. 1999. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 147:791–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalongo, W., M. Jagannadham, and E. Stellwagen. 1993. Kinetic analysis of the hydrodynamic transition accompanying protein folding using size exclusion chromatography. 2. Comparison of spectral and chromatographic kinetic analyses. Biopolymers. 33:135–145. [DOI] [PubMed] [Google Scholar]
- Takagishi, Y., S. Oda, S. Hayasaka, K. Dekker-Ohno, T. Shikata, M. Inouye, and H. Yamamura. 1996. The dilute-lethal (d l) gene attacks a Ca2+ store in the dendritic spine of Purkinje cells in mice. Neurosci. Lett. 215:169–172. [DOI] [PubMed] [Google Scholar]
- Tang, F., E.J. Kauffman, J.L. Novak, J.J. Nau, N.L. Catlett, and L.S. Weisman. 2003. Regulated degradation of a class V myosin receptor directs movement of the yeast vacuole. Nature. 422:87–92. [DOI] [PubMed] [Google Scholar]
- Westbroek, W., J. Lambert, P. Bahadoran, R. Busca, M.C. Herteleer, N. Smit, M. Mommaas, R. Ballotti, and J.M. Naeyaert. 2003. Interactions of human Myosin Va isoforms, endogenously expressed in human melanocytes, are tightly regulated by the tail domain. J. Invest. Dermatol. 120:465–475. [DOI] [PubMed] [Google Scholar]
- Wu, X., B. Bowers, Q. Wei, B. Kocher, and J.A. Hammer III. 1997. Myosin V associates with melanosomes in mouse melanocytes: evidence that myosin V is an organelle motor. J. Cell Sci. 110:847–859. [DOI] [PubMed] [Google Scholar]
- Yin, H., D. Pruyne, T.C. Huffaker, and A. Bretscher. 2000. Myosin V orientates the mitotic spindle in yeast. Nature. 406:1013–1015. [DOI] [PubMed] [Google Scholar]