Figure 2.
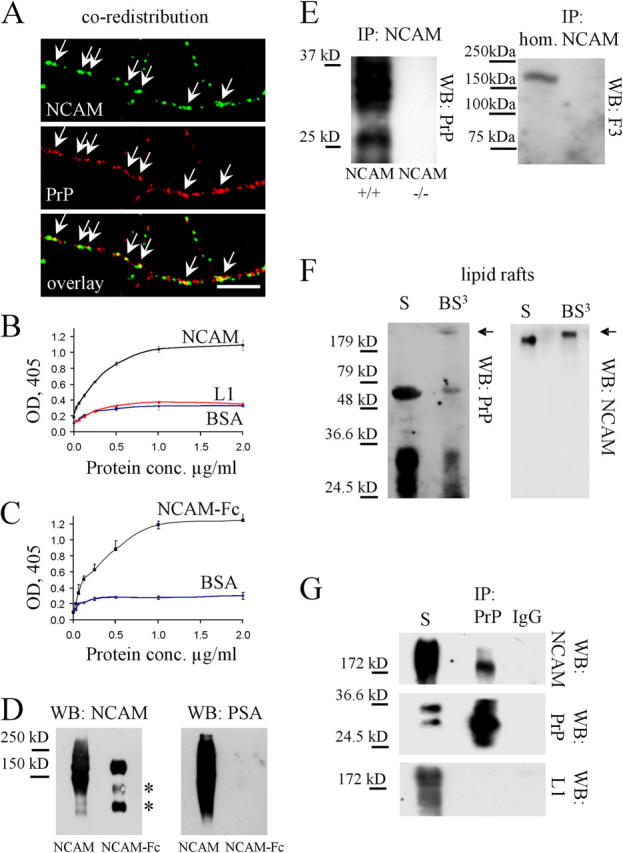
NCAM directly interacts with PrP. (A) Neurons incubated live with antibodies against NCAM to induce clustering of NCAM at the cell surface were fixed and labeled with antibodies against PrP. PrP partially redistributed to NCAM clusters (arrows). Bar, 10 μm. (B) Increasing concentrations of NCAM and L1, purified from mouse brain, were assayed by ELISA for their binding to plastic-bound PrP-Fc (5 μg/ml). Binding of NCAM to BSA (3 mg/ml) served as a control. Mean values (OD405) ± SEM (n = 6) are shown. (C) Increasing concentrations of NCAM-Fc were assayed by ELISA for their binding to plastic-bound PrP-AP (5 μg/ml). Binding of NCAM-Fc to BSA (3 mg/ml) served as a control. Mean values (OD405) ± SEM (n = 6) are shown. (D) NCAM from mouse brain and NCAM-Fc were immunoblotted (WB) with antibodies against NCAM and polysialic acid (PSA). Note that PSA immunoreactivity is found only on NCAM purified from mouse brain. The two bottom bands represent degradation products of NCAM-Fc (asterisk). (E) NCAM immunoprecipitates from the NCAM+/+ mouse brain were immunoblotted (WB) with PrP or F3 antibodies. NCAM−/− brains served as a control for PrP immunoprecipitation. Brain homogenate (hom.) was also probed for F3. PrP, but not F3, coimmunoprecipitated with NCAM. (F) Lipid rafts (S) or lipid rafts treated with BS3 (BS3) were immunoblotted with PrP antibodies (WB: PrP). Then, the membrane was stripped and labeled with NCAM antibodies (WB: NCAM). Note a PrP-immunoreactive band above 200 kD in the cross-linked material that overlaps with a shifted NCAM immunoreactive band (arrows). (G) Total membranes (S) or PrP immunoprecipitates from total membranes treated with BS3 (IP: PrP) were immunoblotted (WB) with antibodies to NCAM, PrP, and L1. Immunoprecipitation performed with nonimmune IgG served as a control (IP: IgG). Note that NCAM, but not L1, coimmunoprecipitated with PrP.
