Figure 2.
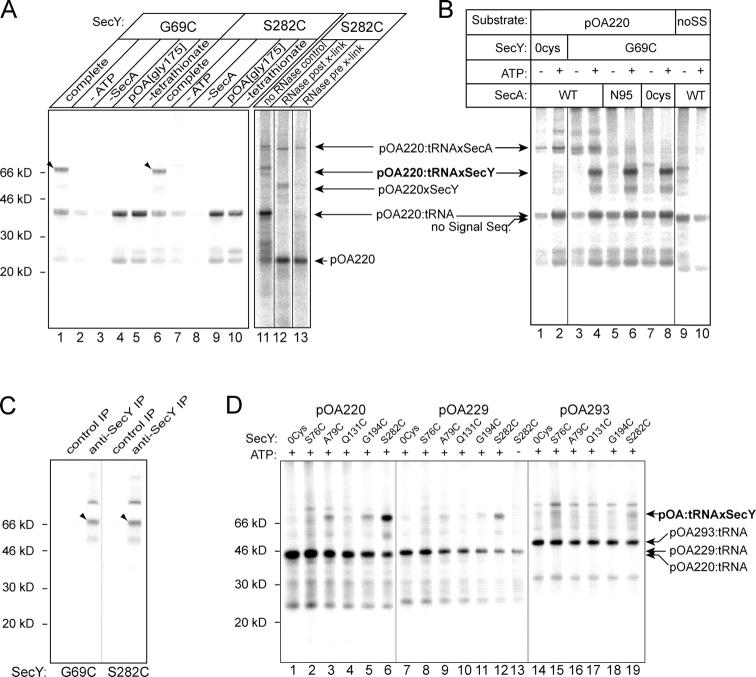
Cysteines at positions 69 and 282 of SecY form a disulfide bond with pOA220:tRNA. (A) SecY mutants G69C and S282C were purified, reconstituted into proteoliposomes, incubated with cysteine-free SecA and 35S-pOA220:tRNA in the presence of ATP, and treated with 100 μM tetrathionate to facilitate disulfide bond formation. “Complete” contains all components; “−” indicates the omitted component. “pOA[Gly175]” is pOA220 with a glycine at position 175 instead of cysteine. Where indicated, samples were treated with RNase before or after cross-linking (pre- and post-X-link). Arrows point to the positions of the substrates and to cross-links with SecY and SecA. The cross-links to SecY are also highlighted by arrowheads. (B) Cross-linking was performed with pOA220 or substrate lacking the signal sequence (deletion of the NH2-terminal 21 aa of pOA220; noSS). SecY mutants lacked cysteines (0Cys) or contained a single cysteine at position 69 (G69C). SecA was either wild type (WT), or lacked three (N95) or all four (0Cys) cysteines. (C) Cross-linked samples were denatured and immunoprecipitated (IP) with affinity-purified polyclonal anti-SecY or control antibodies. (D) 35S-pOA:tRNA fragments of 220, 229, or 293 aa were translocated into proteoliposomes with SecY that contains no cysteines (0cys), or a single cysteine at positions 76, 79, 131, 194, or 282.