Figure 1.
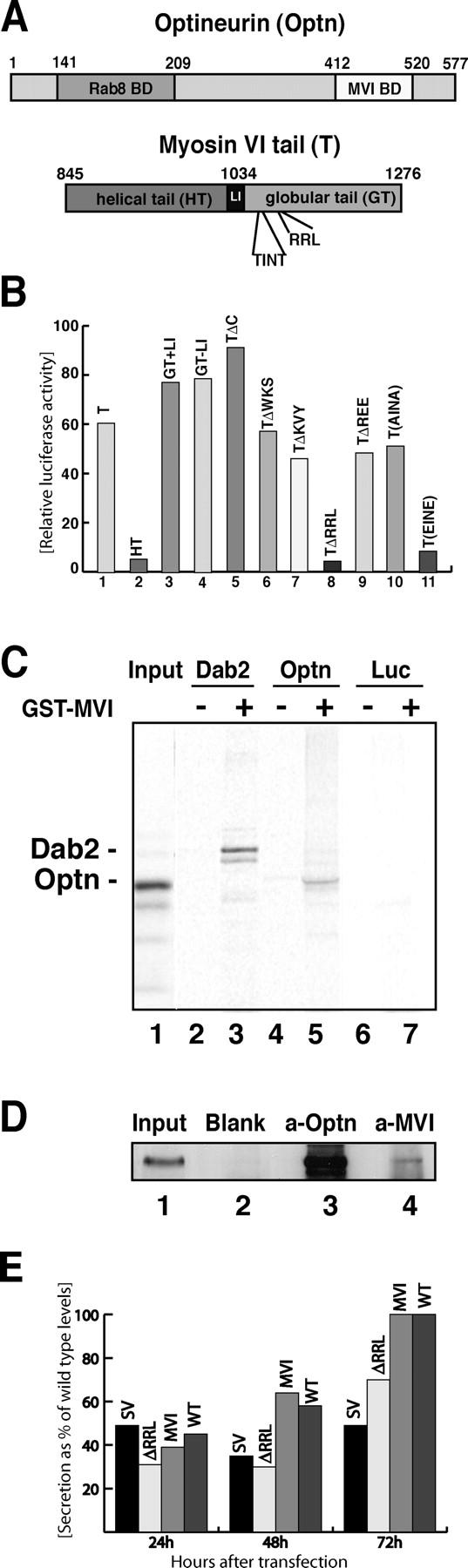
Optineurin is a myosin VI–binding partner. (A) A carton showing the major regions in optineurin and myosin VI tail. Optineurin contains an NH2-terminal Rab8-binding domain (aa 141–209; Hattula and Peranen, 2000) and a COOH-terminal myosin VI–binding domain (aa 412–520). Myosin VI tail has a proposed helical tail domain (HT) and a globular tail (GT), which contains the optineurin-binding site RRL in position 1107–1109. The putative phosphorylation site TINT is at position 1088–1091. (B) Using the ProQuest yeast two-hybrid screen optineurin was identified as a myosin interacting protein. The interaction was verified using the mammalian two-hybrid system. To narrow down the optineurin-binding site on the myosin VI tail the whole tail (T; column 1), the helical tail (HT; column 2) or the globular tail (GT) with or without the large insert (GT+LI or GT-LI; columns 3 and 4) were fused to the Gal4 DNA-binding domain and coexpressed with full-length optineurin fused to the VP16 activating domain in CHO cells. Luciferase activity is shown relative to control cells containing only the myosin VI construct with the empty prey vector. Deletion of myosin VI tail at residue 1117 (TΔC; column 5) did not abolish binding. Mutating three adjacent amino acids each (WKS, KVY, RRL, and REE to AAA) revealed RRL as the optineurin-binding site (columns 6–9). Two threonine residues in positions 1088 and 1091 (TINT) were mutated to either alanine (AINA) or glutamate (EINE; columns 10 and 11). (C) Optineurin binds to purified myosin VI tail. A pull down was performed using in vitro–translated optineurin and GST-myosin VI tail. 35S-labeled in vitro–translated full-length optineurin (lanes 4 and 5), Dab2 as a positive control (lanes 2 and 3) or luciferase as a negative control (lanes 6 and 7) were incubated with either 5 μg GST alone (lanes 2, 4, and 6) or with 5 μg GST-myosin VI tail (lanes 3, 5, and 7). Lane 1 shows 50% of the input used for the pulldown with [35S]optineurin in lane 5. The band below Dab2 is possibly caused by degradation (lane 3). (D) Co-immunoprecipitation of endogenous myosin VI and optineurin from the cytosol of A431 cells. Immunoprecipitation was performed under native conditions using no antibody, only protein A beads as a control (lane 2, blank), an antibody to full-length optineurin (lane 3, a-Optn) or a pAb to the tail of myosin VI (lane 4, a-MVI). Lane 1 is equivalent to 5% of the input used for each immunoprecipitation. Immunoprecipitates were run out on a 10% PAGE gel, blotted, and analyzed using an antibody to optineurin. (E) Constitutive secretion in Snell's waltzer fibroblasts cannot be rescued by overexpression of the mutant myosin VI lacking the optineurin-binding site (ΔRRL). Rescue experiments were performed by generating Snell's waltzer cell lines stably expressing only GFP (SV), the mutant myosin VI without the optineurin-binding site (ΔRRL), or wild-type whole myosin VI (MVI). Levels of secretion were compared with wild-type cells stably expressing only GFP (WT). To measure constitutive secretion, a construct, which expresses a SEAP was transfected into the various Snell's waltzer cell lines and the wild-type fibroblasts. The amount of alkaline phosphatase secreted into the media was measured 24, 48, and 72 h after transfection and plotted as a percentage of total maximal secretion in wild-type cells after 72 h. Transfection efficiency was normalized by cotransfection of a control plasmid expressing a red fluorescent protein. The data from two separate experiments run in triplicate are shown.
