Figure 5.
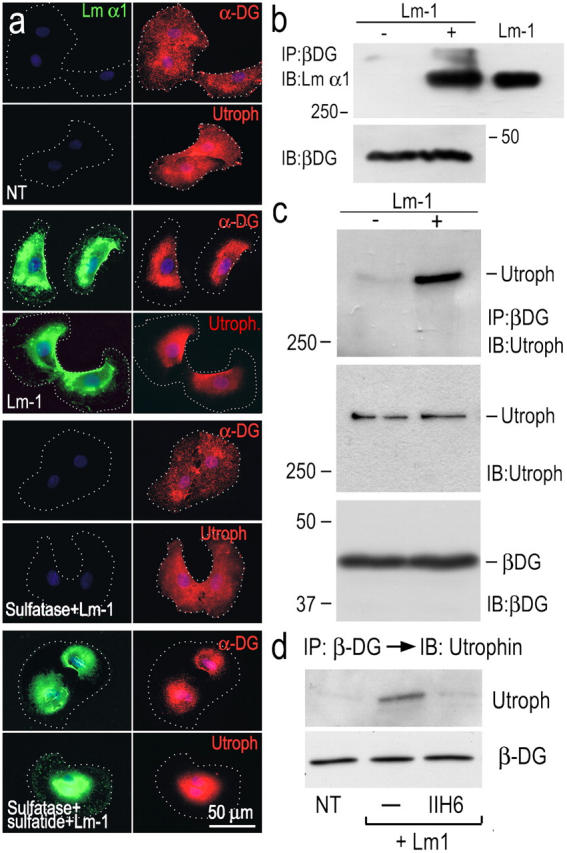
Lm-1 binding to sulfatide recruits DG and utrophin in SCs. (a) SCs, untreated (NT), treated with 10 μg/ml Lm-1 (for 1 h), treated with 50 U/ml arylsulfatase followed by Lm-1, or treated with arylsulfatase and then loaded with sulfatide followed by Lm-1, were immunostained for the indicated components (dots indicate cell borders). α-DG and utrophin condensation (and Lm colocalization) were prevented if the cells were treated with arylsulfatase. (b) Lm-1–DG association. SCs untreated (−) or treated (+) with 10 μg/ml Lm-1 (for 1 h) were washed and extracted with 1% Triton X-100. The cell lysates were immunoprecipitated (IP) with anti–β-DG antibody and probed for Lm-α1 chain. The input cell lysates were also immunoblotted (IB) with anti–β-DG antibody. Exogenous Lm was detected in the precipitates when added to the medium. (c and d) Recruitment of utrophin to DG. The immunoprecipitates formed with anti–β-DG antibody were subjected to immunoblotting with utrophin-specific antibody (c). Utrophin recruitment was blocked by antibody IIH6 (d).
