Figure 6.
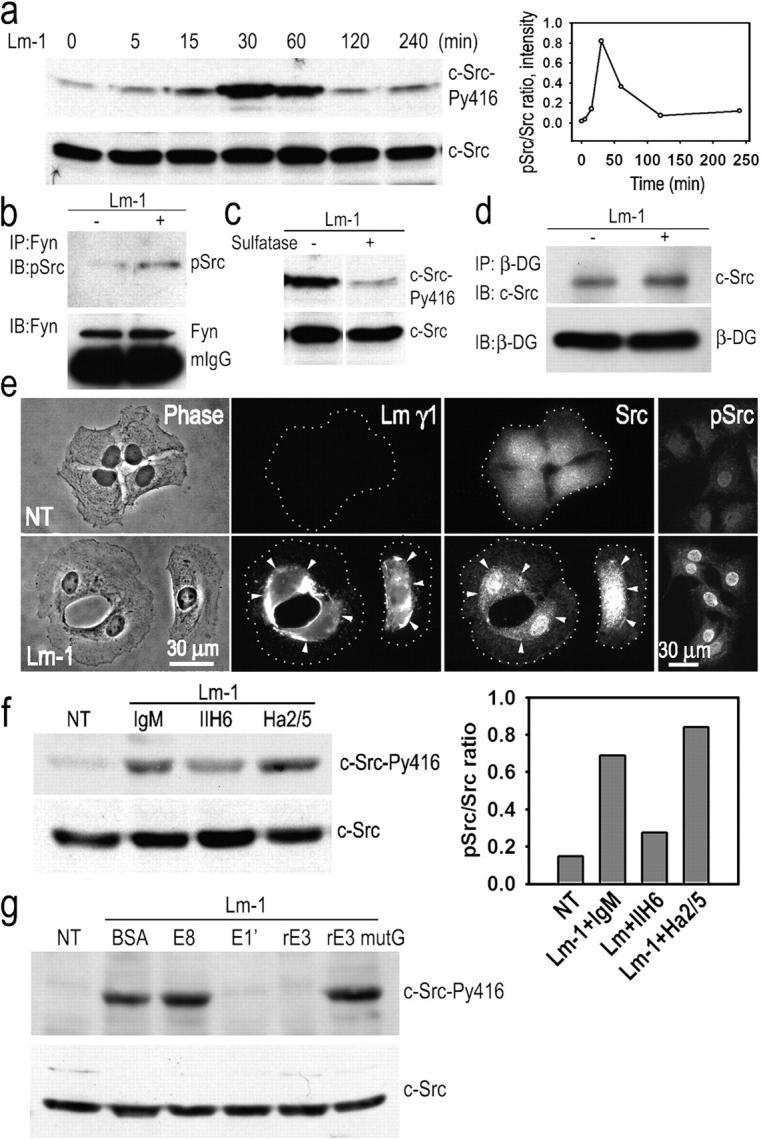
Schwann cell c-Src is activated in response to Lm-1. (a) Transient Src activation in response to Lm: SCs were incubated with 10 μg/ml Lm-1, harvested at the indicated times, lysed, and analyzed for c-Src-PY416 and c-Src. Time course immunoblot and densitometry plot of pSrc/total Src ratio are shown. (b) Fyn activation in response to Lm: lysates from SCs treated as above for 1 h were immunoprecipitated with Fyn-specific antibody followed by immunoblotting with phospho-Src (PY416) antibody that also detects pFyn. (c) Src activation depends on the presence of gal-sulfatide. SCs were treated with Lm-1 as above for 1 h in the presence (+) or absence (−) of 50 U/ml arylsulfatase and analyzed for c-Src phosphorylation. (d) c-Src coimmunoprecipitates with β-DG. SCs untreated or treated with 10 μg/ml Lm-1 for 1 h were extracted with 1% Triton X-100–Tris buffer. Cell lysates were immunoprecipitated with anti–β-DG antibody and the immunoprecipitates were subjected to immunoblot analysis with c-Src-–specific antibody. (e) SCs were untreated or treated with 10 μg/ml Lm-1 for 1 h and immunostained for Lm-γ1, Src, and Src-PY416 (pSrc). Diffusely distributed Src immunofluorescence coalesces into dense plaques that overlap with Lm immunofluorescence after Lm-1 treatment, whereas most of Src-PY416 is associated with the nucleus (arrowheads indicate colocalizations of antibody immunofluorescence between paired panels, establishing the relationship at various points). (f) Anti–α-DG antibody IIH6 inhibits Src phosphorylation, whereas anti–β1-integrin (Ha2/5) does not. The bar graph shows the phospho-Src/total Src ratio based on the immunoblot densitometry. (g) Lm-1 fragments E1′ and E3, but not E8 or E3 mutG (which lack a sulfatide-binding sequence), block c-Src phosphorylation. SCs were incubated for 1 h with Lm-1 in the presence of 100 μg/ml BSA, 250 μg/ml E8, 250 μg/ml E1′, 100 μg/ml rE3, or 100 μg/ml rE3 mutant G. Cells were washed, lysed in 1% SDS-Tris buffer, and immunoblotted.
