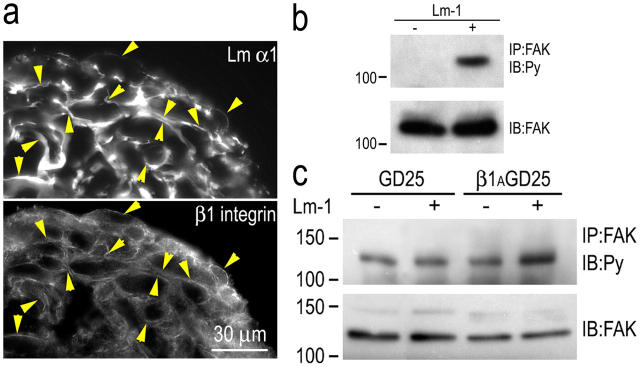
Figure 9.
β1-integrin association with Lm and FAK phosphorylation. SCs or ES cell–derived fibroblasts were cultured as aggregates in poly-HEMA–coated dishes for 24 h in the presence or absence of 10 μg/ml Lm-1. (a) Cryosections of SC aggregates were immunostained for Lm-α1 and β1-integrin. The integrin colocalized with Lm at cell edges. Arrowheads indicate colocalizations of antibody immunofluorescence between paired panels, establishing the relationship at various points. (b) Lm-1 induces FAK phosphorylation in suspended SCs. SC aggregates were maintained overnight in the absence (−) or presence (+) of 10 μg/ml Lm-1. The aggregates were washed, lysed in 1% Triton X-100–Tris buffer, immunoprecipitated with anti-FAK antibody (IP: FAK), and immunoblotted with antiphosphotyrosine (Py) antibody. The immunoblot of total FAK was used as the control. (c) Lm-1 induction of FAK phosphorylation in fibroblasts is dependent on β1-integrin. GD25 ES cell–derived fibroblasts and GD25 cells transduced with a β1-integrin expression vector (control) were loaded with sulfatide and grown in suspension overnight in the absence (−) or presence (+) of 10 μg/ml Lm-1. Cell extracts were analyzed for tyrosine phosphorylation of FAK and total FAK as described above. Lm-1 treatment increased FAK phosphorylation level in the control β1AGD25 cells but not the β1-integrin–deficient GD25 fibroblasts.