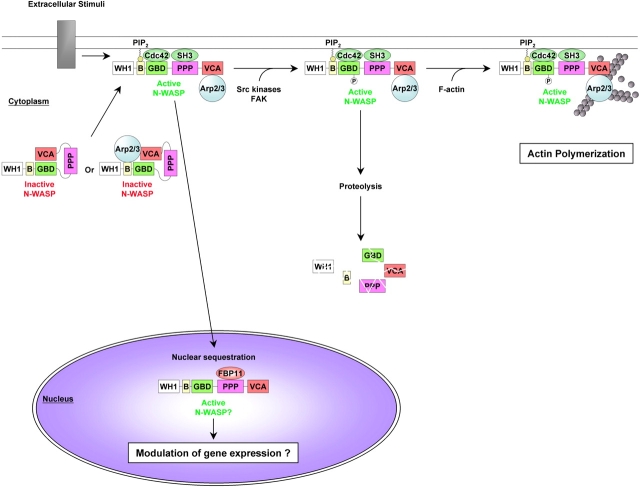
Figure 1.
Model for N-WASP regulation. Under resting conditions, N-WASP is maintained in an inactive state in the cytosol. Autoinhibition is via interaction of the GBD and the C domain of the VCA module. Alternatively, direct interaction with the basic-rich region could inhibit the activity of a prebound Arp2/3 complex. In response to extracellular stimuli, N-WASP autoinhibition is relieved by the binding of GTP-bound Cdc42 and PtdIns(4,5)P2 (PIP2) or SH3 containing proteins (SH3). In the open conformation, N-WASP can be imported into the nucleus, where it may be retained by FBP11 and regulate gene expression. Alternatively, active N-WASP can stay in the cytosol. Phosphorylation by tyrosine kinases enhances the ability of N-WASP to activate the Arp2/3 complex in cooperation with F-actin, prevents its nuclear import, and may induce—in certain models—its degradation through the proteasome pathway.