Figure 2.
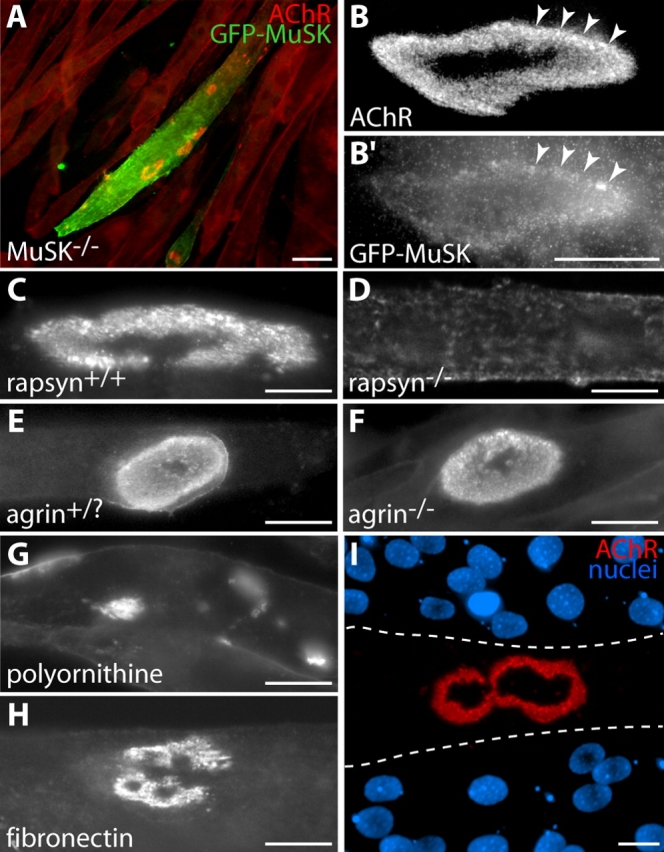
Requirements for formation of branched AChR aggregates on laminin-coated substrates. (A–B) MuSK is required. Myotubes from a MuSK−/− cell line form no AChR aggregates when cultured on laminin-coated substrates. AChRs are instead distributed uniformly over their surface (cells stained uniformly red with Btx). When transfected with GFP-MuSK (green), such cells form receptor aggregates (red) with complex morphology (A). High power view shows colocalization of these aggregates (B) with GFP-MuSK (B′). Arrowheads mark corresponding points in B and B′. (C and D) Rapsyn is required. Myotubes from a control cell line form complex AChR aggregates when cultured on laminin (C), but myotubes from a rapsyn−/− cell line form no clusters (D). (E and F) Agrin is not required. Primary myotubes from controls (E, agrin+/?, meaning heterozygous or wild type) and agrin−/− (F) mice both form complex clusters on laminin-coated substrates. (G) Polyornithine-coated substrates do not support complex AChR aggregate formation in C2 cells. AChR aggregates in these cultures are plaque shaped. (H) Laminin is not required. C2 myotubes form branched aggregates on fibronectin-coated substrates, although they are generally smaller and less complex than those that develop on laminin-coated substrates. (I) When C2 myotubes are mechanically detached from laminin-coated substrata, postsynaptic “ghosts” labeled with Btx (red) remain attached to the substrate. Nuclear staining with DAPI (blue) shows that there is no myotube over the receptor aggregate. Dotted lines indicate approximate borders of the former myotube. Bars: (A) 100 μm; (B′–I) 20 μm.
