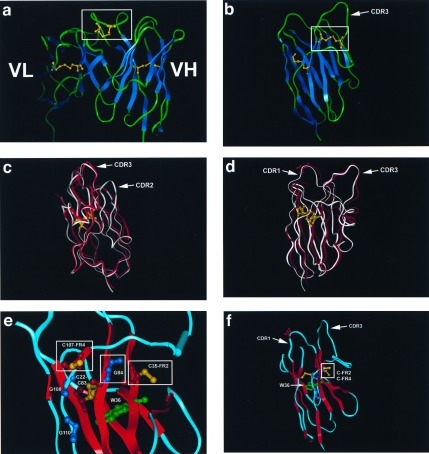
Figure 4.
Modeling of NAR V and comparison with camel and human VH structures. (a) The VH/VL of the KOL human crystal (18, 23) showing the disulfide bridge in the CDR3 VH (boxed). Note that the VL (left) binding site is obscured by the VH CDR3. Canonical intradomain disulfide bonds between the two β sheets of the H and L chains are displayed in yellow (not boxed). The boxed disulfide bridge (also yellow) is postulated to be similar to bonds that would form in NAR Type I CDR3. (b) Crystal structure of the unusual camel VH (15). The disulfide bridge between CDR1 and 3 is boxed, and the canonical intradomain disulfide bridge is displayed. The disulfide bond displayed here is postulated to be similar to those formed by CDR1 and 3 Cys residues in NAR Type II proteins. (c and d) Model of the Type I NAR V domain (red) superimposed on the camel VH (white). Note the high similarity of the two structures except in the CDR2 region, which in NAR is very small and may connect the two β sheets in a fashion similar to Ig C1 domains (33). The canonical disulfide bridge (yellow) and serine (red) in NAR CDR1 (S-28, Fig. 2) are displayed for bearings. (e and f) Potential disulfide bond formed in NAR between unconventional Cys (boxed) in FR2 (C-35) and FR 4 (C-107); see Figs. 2 and 3 a and c. The side chains of these two Cys project into the region in which conventional VH and VL interact. We propose that the small size of the highly conserved glycine at position 84 (boxed G-84, Figs. 2 and 3c) permits C-35 and C-107 to disulfide-bond; it is important to emphasize that this glycine is conserved only in Type I NAR sequences and is rarely found in Ig/TCR of other vertebrates or even in Type II NAR (3). We speculate that NAR is more compact, and, because of irregular H bonding in the G strand (FR4) of the model, it is possible that a disulfide bond can be formed between C-35 and C-107. The canonical disulfide bond (C-22, C-83) and the invariant tryptophan (W-36) forming the core of the NAR domain, as well as the invariant Gly-Xaa-Gly (ref. 18; G-108, G-110), are displayed.