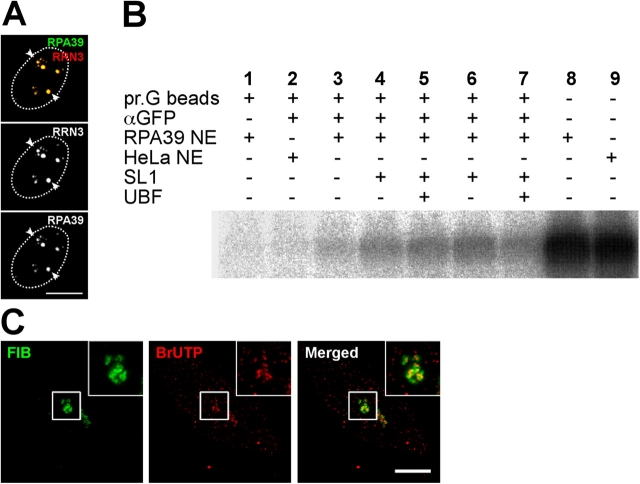
Figure 1.
Characterization of YFP-RPA39 cell lines. (A) HeLaYFP-RPA39 cells were fixed 10 h after transient transfection with CFP-RRN3. Arrowheads indicate the colocalization of punctate structures labeled by YFP-RPA39 and CFP-RRN3 within nucleoli. The nuclear boundaries are denoted by dotted ovals. Bar, 5 μm. (B) In vitro transcription assay. Nuclear extracts prepared from HeLaYFP-RPA39 and parental HeLa cells were tested for specific transcription initiation activity on the rDNA promoter, which is shown by the presence of nascent rDNA transcript (lanes 8 and 9). Lanes 1–7 assay transcription from RNA Polymerase I complexes immunoprecipitated with anti-GFP. Anti-GFP-immunoprecipitated complexes from HeLaYFP-RPA39 nuclear extracts were transcriptionally active upon the addition of rRNA gene promoter template DNA and ribonucleoside triphosphates (lane 3). Addition of SL1 alone (lanes 4 and 6) or SL1 and UBF (lanes 5 and 7) further stimulated transcription. In the absence of anti-GFP antibody no transcription was observed (lane 1) and in the presence of anti-GFP-protein G beads but absence of a GFP tag (parental HeLa nuclear extract) no transcription was observed (lane 2). (C) Pulse-labeled rRNA transcripts transiently colocalize within the DFC after a 6-min chase. The left panel shows the localization of DFC labeled by YFP-FIB in stable HeLaYFP-FIB cell lines and the middle panel illustrates the localization of accumulated BrUTP at 6 min and the pattern of incorporation within nucleoli are shown in detail in the inserts. Bar, 5 μm.