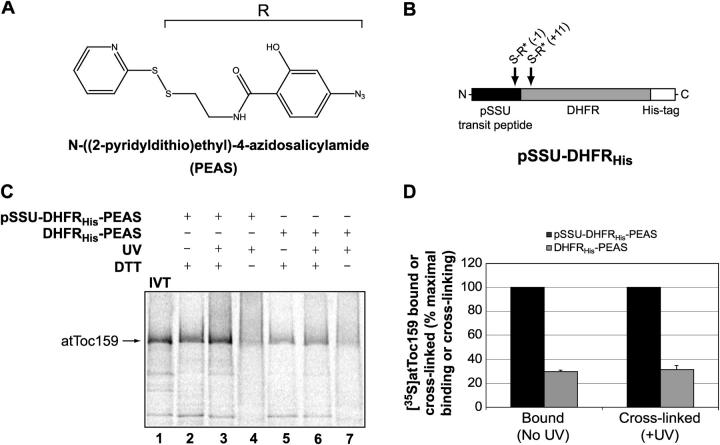
Figure 5.
Chemical cross-linking of atToc159 to the transit peptide of the small subunit of Rubisco. (A) Structure of the heterobifunctional PEAS cross-linker. The photoactivatable phenyl azido group and linker arm that are transferred to a cysteine residue in a disulfide exchange reaction are labeled as “R.” (B) Schematic representation of the pSSU-DHFRHis construct used in the cross-linking reactions. Arrows point to cysteines at positions −1 and +11 of pSSU-DHFRHis that, when fully reduced, undergo a disulfide exchange with PEAS (indicated by R*). (C) [35S]atToc159 was incubated with immobilized pSSU-DHFRHis-PEAS or DHFRHis-PEAS. After the incubation, reactions were treated without (−) or with (+) UV light to activate the cross-linker. Resin-bound proteins were eluted from the Ni-NTA and treated with (+) or without (−) DTT before being resolved by SDS-PAGE. [35S]atToc159 was detected in dried gels using a phosphorimager. Lane 1 contains 30% of the [35S]atToc159 in vitro translation product (IVT) added to each reaction. (D) [35S]atToc159 bound or cross-linked to pSSU-DHFRHis-PEAS or DHFRHis-PEAS in samples treated with DTT was quantitated using a phosphorimager. Data are presented as the percentage of maximal binding or cross-linking. Quantitation of the data from two replicates is shown.