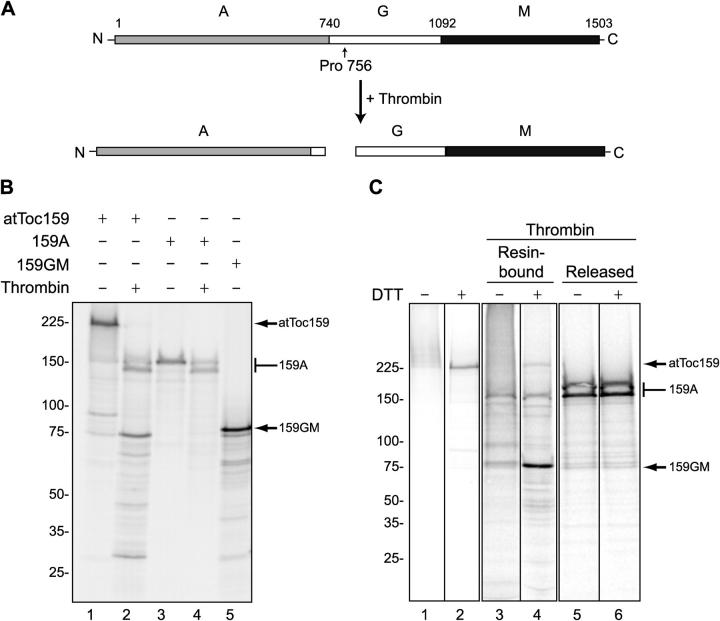
Figure 6.
Transit peptides cross-link to the GM domain of atToc159. (A) Schematic representation of the proteolysis strategy for mapping the transit peptide–binding site on atToc159. Thrombin is predicted to cleave atToc159 between Pro756 and Asp757. (B) [35S]atToc159, [35S]atToc159A, and [35S]atToc159GM were treated without (−) or with (+) thrombin for 1 h at 37°C and resolved by SDS-PAGE. (C) pSSU-DHFRHis was modified with PEAS as described in the legend to Fig. 5, immobilized on Ni-NTA resin, and incubated with [35S]atToc159 in the presence of GTP. After cross-linking, reactions were treated without (lanes 1 and 2) or with (lanes 3–6) thrombin and were separated into resin-bound and released fractions. The samples were then treated without (−) or with (+) DTT, separated by SDS-PAGE, and analyzed using a phosphorimager. The positions of mol wt markers (kD) are indicated to the left, and atToc159, atToc159A (159A), and atToc159GM (159GM) to the right of each figure. Black lines indicate grouping of images from different portions of the same gel. Images from different gels are in separate boxes.