Figure 1.
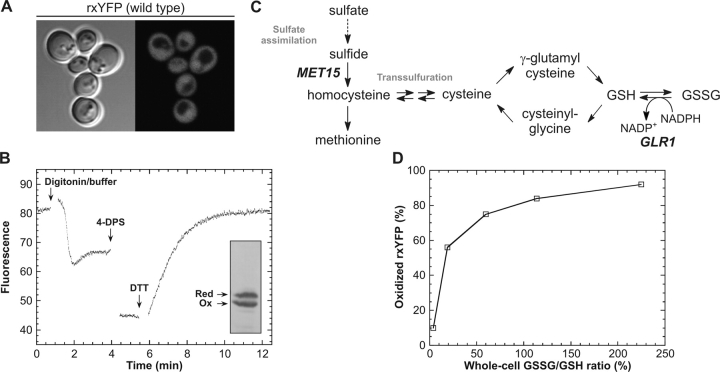
Determining intracellular redox properties of cytosolic rxYFP. (A) Fluorescence distribution in wild-type yeast cells (M4745) expressing cytosolic rxYFP from the PGK1 promoter (fluorescence micrograph recorded using a Leitz DMIRBE microscope). (B) A representative fluorometric redox-determination of rxYFP expressed in a glutaredoxin reductase-deficient yeast mutant (M4860). The culture was pretreated with 0.1% digitonin in strong buffer to permeabilize the cells at neutral pH (see Materials and methods). From the fluorescence of the permeabilized cells and the fluorescence after sequential addition of 4-DPS and DTT to final concentrations of 0.3 mM and 93 mM, respectively, the proportion of oxidized rxYFP was calculated to 56%. Due to dilution of the sample during the procedure, the final fluorescence appears to be lower than would be expected from the reported fraction of oxidized reporter. (Inset) Western blot detection of oxidized and reduced rxYFP in the same culture. Cells were quenched by addition of 5% TCA. Subsequently, free thiols were blocked by treatment with 100 mM NEM, and the oxidized and reduced states of rxYFP were separated by nonreducing SDS-PAGE. Quantification of the immunostained bands yielded 53% oxidized rxYFP. (C) Overview of part of the sulfur metabolism in yeast relevant to the present work. The MET15 and GLR1 gene products catalyze the incorporation of sulfide into homocysteine and the NADPH-dependent reduction of GSSG into GSH, respectively. (D) Relationship between the GSSG/GSH ratios determined on whole-cell extracts and the measured proportion of oxidized rxYFP determined by fluorometric redox titration (values were taken from Table I).