Figure 1.
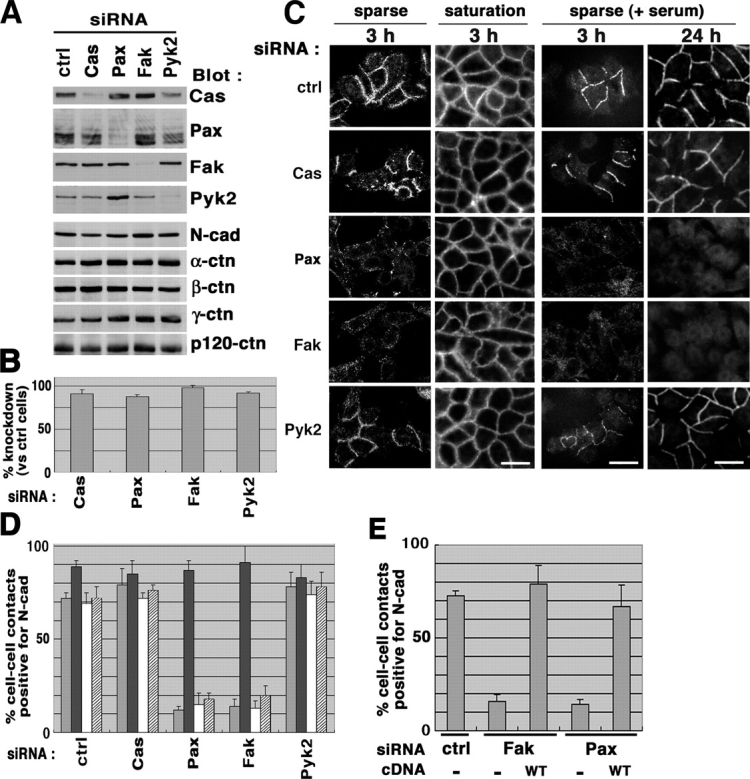
Loss of Fak or paxillin expression affects formation of N-cadherin–based cell–cell adhesions in motile HeLa cells. (A and B) siRNA-mediated knockdown of integrin signaling proteins. HeLa cells were transfected with siRNA duplexes to knockdown of protein expression of p130Cas (Cas), paxillin (Pax), Fak, and Pyk2, as indicated. A control included an irrelevant siRNA duplex (ctrl). Each 20 μg of cell lysates was separated on SDS-PAGE and subjected to immunoblot analyses, as indicated (A); percentages of the knockdown are shown (B). Results are means ± SEM from three independent experiments. Protein levels of N-cadherin (N-Cad) and α-, β-, γ-, and p120-catenin (α-ctn, β-ctn, γ-ctn, and p120-ctn, respectively) were also shown by immunoblotting (A). (C and D) siRNA-treated cells were replated onto collagen at a sparse or saturation density in the absence of serum (C, left two panels), or at a sparse density in the presence of 10% FCS (C, right two panels) as described in Materials and methods. After incubation for 3 or 24 h, cells were fixed and subjected to N-cadherin immunostaining (C). Bars (for all panels in C), 50 μm. Percentages of the N-cadherin–positive cell–cell contact areas are shown (D), in which four different bars, from left to the right, correspond to the four different replating and culture conditions in C, from left to the right, respectively. (E) Restoration by rescue cDNAs. Each rescue cDNAs or empty vector (−) was transfected into the corresponding siRNA-treated cells, and cells expressing exogenous Fak or paxillin were identified, as described in Materials and methods. Percentages of the N-cadherin–positive cell–cell contact areas were measured after a 3-h incubation of cells replated at a sparse density in the absence of serum. In D and E, results are means ± SEM from four independent experiments, in each of which >50 cell–cell contact areas were examined.
