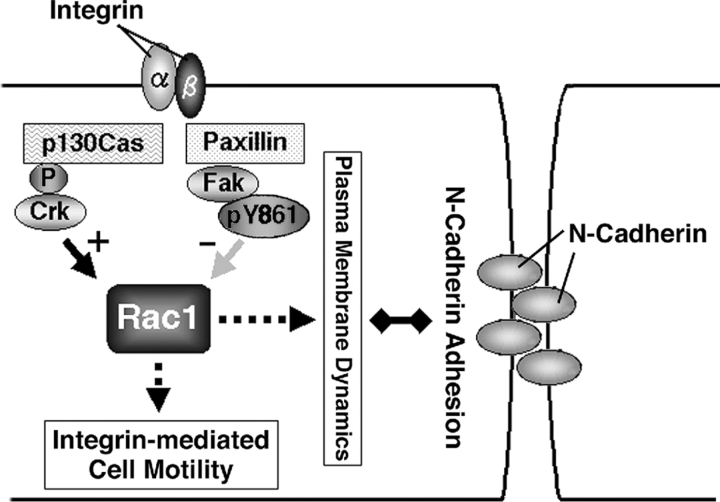
Figure 8.
A hypothetical model showing the roles of Fak and paxillin in motile HeLa cells. Integrin signals have been well documented to include a pathway that leads to activation of Rac1 activity, such as through the p130Cas and Crk pathway. Such activation of Rac1 is closely related to plasma membrane ruffling and enhanced cell motility. Our results suggest that integrin signals also include a pathway that evokes a mechanism to down-regulate Rac1 activity at the cell–cell contact areas of the cell periphery, in which the presence of Fak and paxillin appears to be essential. Paxillin participates in efficient FA recruitment of Fak, and Tyr861 phosphorylation may be important for Fak function. Such localized down-regulation of Rac1 activity appears to be crucial for the maintenance of the normal morphology and normal dynamics of the plasma membrane, the formation of N-cadherin–based cell–cell adhesions, and perhaps also for the maintenance of N-cadherin–based cell–cell adhesions in the collective migration of HeLa cells. Integrin-mediated down-regulation of Rac1 activity upon cell–cell collision may also be important for the contact inhibition of movement.