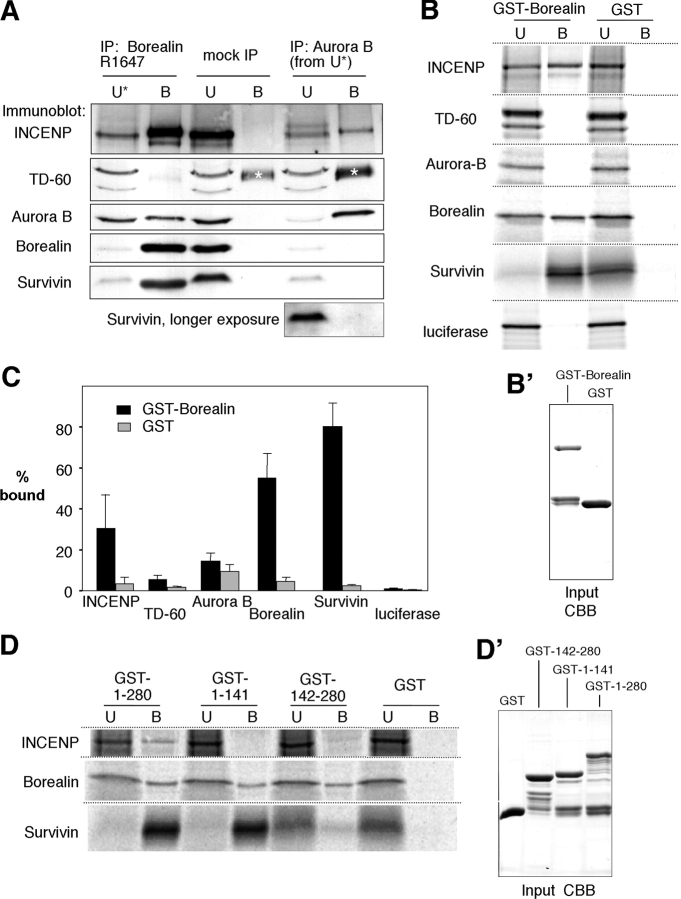
Figure 4.
Borealin interacts with subunits of the chromosomal passenger complex in vivo and in vitro. (A) Chromosomal passengers coimmunoprecipitate with endogenous Borealin. Mitotic HeLa cell lysate was incubated with affinity-purified R1647 (IP: Borealin) or preimmune serum R1647 (mock IP) cross-linked to protein A beads. Chromosomal passenger proteins in the unbound (U) and bound (B) fractions were visualized by immunoblotting. The unbound fraction after immunoprecipitation of Borealin was further incubated with anti-Aurora B antibody (IP: Aurora B) cross-linked to protein A beads. Protein loading per lane is equivalent to mitotic lysate from 1.5 × 105 cells. The asterisk denotes residual rabbit IgG heavy chain coeluted from the beads due to incomplete cross-linking. (B) Borealin binds to Survivin, INCENP, and itself in vitro. Proteins were translated in the presence of [35S]methionine and incubated with bacterially expressed GST-Borealin or GST alone bound to glutathione sepharose beads. Bound (B) and unbound (U) fractions were separated by SDS-PAGE, and the proteins were visualized by phosphorimaging. Luciferase was included to check for nonspecific binding of GST-Borealin. (B') GST-Borealin and GST used in the binding reactions (input) stained with Coomassie brilliant blue. (C) Quantification of the binding experiments shown in B. The bars represent the percentage of total translated protein bound to GST-Borealin (black) or GST (gray; n ≥ 3). (D) The NH2- and COOH-terminal halves of Borealin show differential binding properties in vitro. Experiments were performed as described in B, but included GST fusions of Borealin1–141 and Borealin142–280 in addition to full-length Borealin1–280. Equal loading (input) was checked by CBB staining (D').