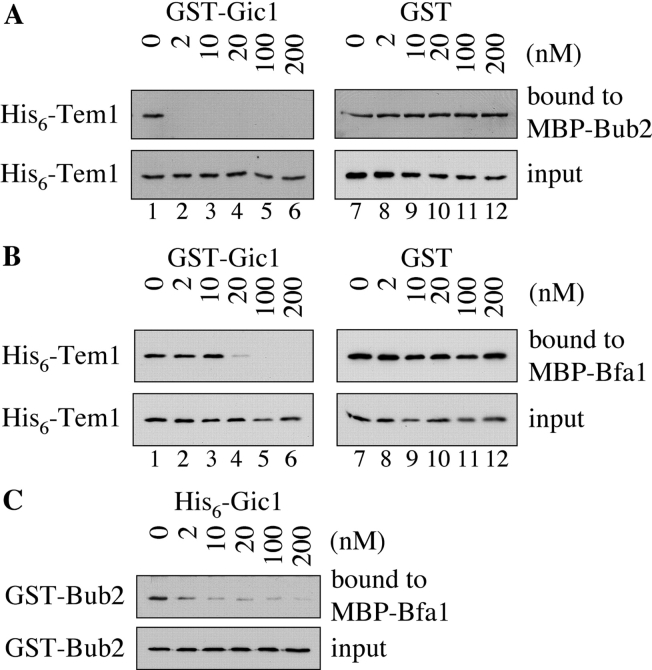
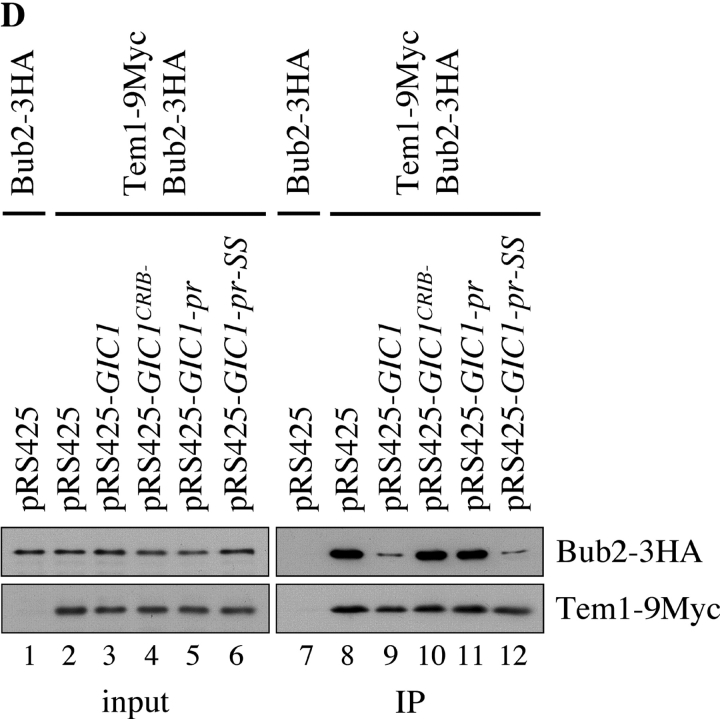
Figure 4.
Gic1 disrupts the formation of the Bfa1–Bub2–Tem1 complex. (A) Gic1 disrupts the binding between Tem1 and Bub2. His6-Tem1 (20 nM final concentration) was incubated with increasing amounts of recombinant GST-Gic1 (0–200 nM) or GST (0–200 nM). The preincubated proteins were added to MBP-Bub2 beads (Bub2 was 20 nM) and incubated for 60 min. After washing, proteins bound to the beads were eluted and examined by immunoblotting using antibodies against Tem1. The fact that 2 nM Gic1 (lane 2) was able to completely neutralize 20 nm Tem1 indicates either that Tem1 is partially inactive, the protein concentration of Tem1 is underestimated or that Gic1 has multiple binding sites for Bub2 or Tem1. (B) Gic1 can disrupt binding of Tem1 to Bfa1. The experiment was carried out as in A but with MBP-Bfa1 beads. (C) Gic1 can disrupt binding between Bfa1 and Bub2. GST-Bub2 (20 nM) was incubated with increasing amounts of bacterial His6-Gic1 (0–200 nM). Proteins were added to MBP-Bfa1 beads (20 nM) and incubated for additional 60 min. After washing the beads bound proteins were eluted and examined by immunoblotting using antibodies against Bub2. (D) Gic1 can disrupt the Tem1–Bub2 complex in vivo. TEM1 BUB2-3HA (lanes 1 and 7) and TEM1-9Myc BUB2-3HA cells (lanes 2–6 and 8–12) carrying pRS425, pRS425-GIC1, pRS425-GIC1 crib−, pRS425-GIC1-pr, or pRS425-GIC1-pr-SS were lysed. Equal amounts of protein extract were incubated with anti-Myc antibodies. Immunoprecipitates were analyzed by immunoblotting with antibodies against the Myc and HA epitopes.