Figure 6.
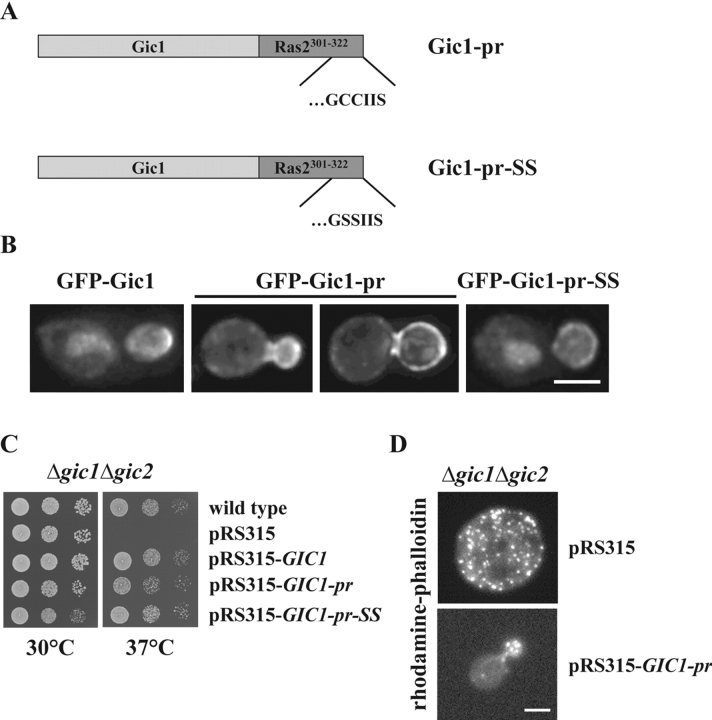
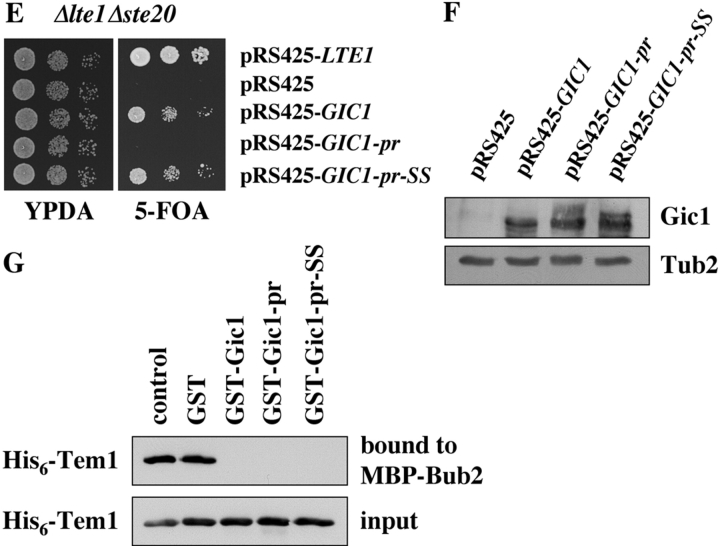
A permanently membrane-bound Gic1-pr promotes actin organization but not mitotic exit. (A) Schematic representation of the Gic1-Ras2301-322 fusion proteins. Full-length Gic1 (light gray) was fused to the most COOH-terminal part of wild-type and a mutated Ras2 (dark gray). The CCIIS region (CAAX box) of wild-type Ras2 or the mutated SSIIS (Cys residues 218 and 219 were replaced by Ser) are indicated. The C218S C219S mutations prevent palmitoylation and prenylation and thereby anchorage of Ras2 at the plasma membrane. (B) Localization of Gic1-pr and Gic1-pr-SS. Gal1-GFP-GIC1, Gal1-GFP-GIC1-pr, and Gal1-GFP-GIC1-pr-SS cells were grown in raffinose medium to mid-log phase. The Gal1 promoter was then induced for 1 h by the addition of galactose. Localization of fusion proteins was determined by deconvolution fluorescence microscopy. (C) Membrane-bound Gic1 rescued the growth defect of Δgic1 Δgic2 cells. A serial dilution of wild-type and Δgic1 Δgic2 cells harboring the indicated centromeric pRS315 derivatives (Sikorski and Hieter, 1989) were spotted onto YPDA plates. Plates were incubated at 30°C and 37°C. (D) GIC1-pr complements the actin polarization defect of Δgic1 Δgic2 cells. Δgic1 Δgic2 cells with either pRS315 or pRS315-GIC1-pr were grown in selective medium for 2 h at 37°C. Cells were fixed and the F-actin cytoskeleton was stained with rhodamine-phalloidin. (E) Membrane-bound Gic1 did not suppress lethality of Δlte1 Δste20 cells. Δlte1 Δste20 pRS316-LTE1 cells were transformed with the indicated multicopy pRS425 plasmids. Serial dilutions (1:10) of the cells were spotted onto YPDA and 5-FOA plates. Cells were incubated at 30°C. (F) Cells of E were grown in selective medium and analyzed by immunoblotting with antibodies against Gic1 and Tub2. (G) Gic1-pr and Gic1-pr-SS disrupt the binding between Tem1 and Bub2 in vitro. Experiment was performed as in Fig. 4 A using purified, recombinant proteins. Concentration of all proteins was 20 nM. Bars, 5 μm.