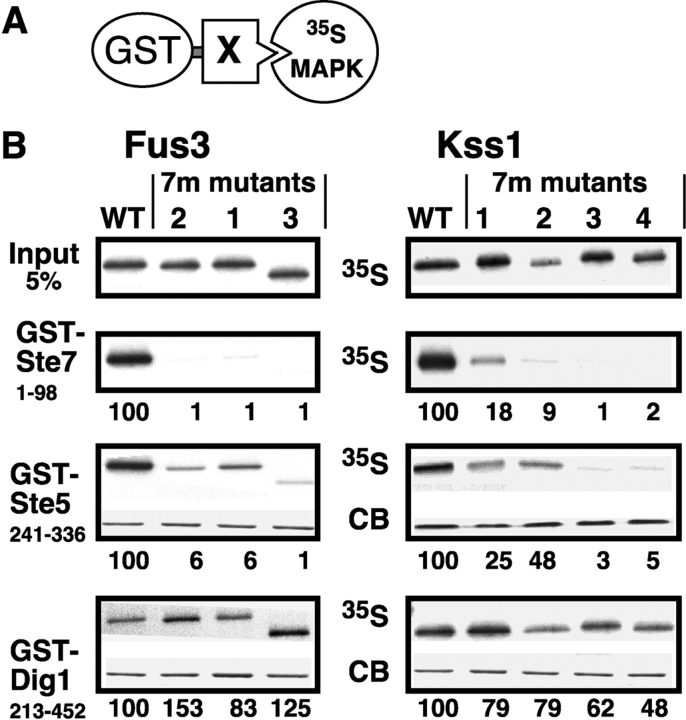
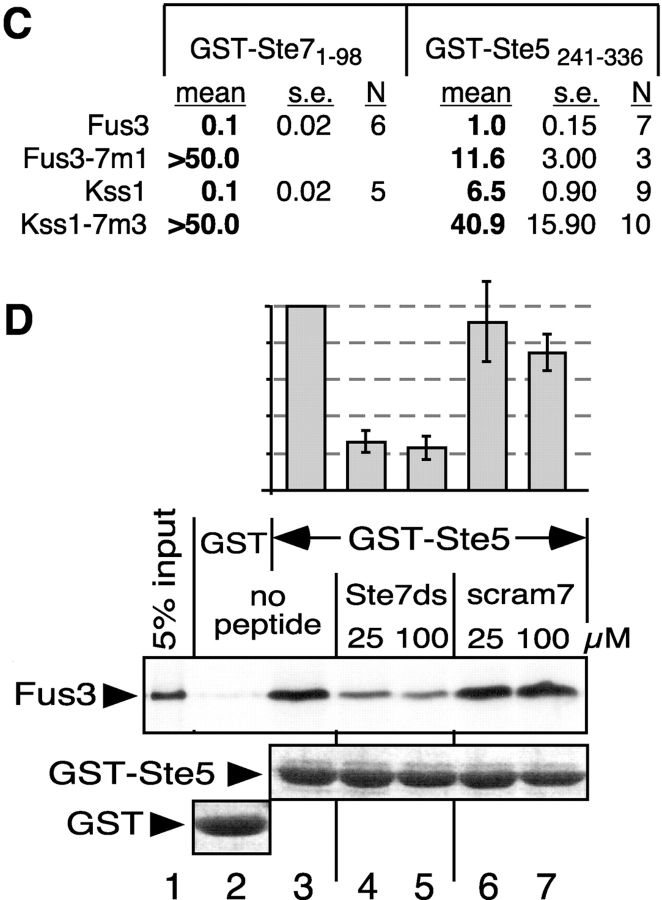
Figure 2.
Impaired binding of MAPK CD/7m-region mutants to Ste7 and Ste5. (A) MAPK-binding domains of Ste7, Ste5 and Dig1 were fused to GST and tested for binding to 35S-labeled Fus3, Kss1, or mutants thereof. (B) In vitro binding assay. MAPK proteins (∼1 pmole; ‘Input’ (top) shows 5% of this amount) were incubated with 100 ng of GST-Ste71-98, 2 μg of GST-Ste5241-336, or 10 μg of GST-Dig1213-452. Glutathione-Sepharose beads were then added, and the resulting bead-bound protein complexes were isolated, resolved by 10% SDS-PAGE, and analyzed by staining with Coomassie blue (CB) for visualization of the bound GST fusion protein, and by autoradiography for visualization of the bound radiolabeled (35S) protein. No Coomassie blue staining is shown for GST-Ste7 because the small amount used (100 ng) was difficult to photograph (but was verified by eye). The numbers below show the normalized relative binding, with the binding of the wild-type set at 100. The percent of the input of wild-type Fus3 bound to Ste7, Ste5, and Dig1 was 14.0, 12.6, and 0.6, respectively. For Kss1: 9.0, 3.7, and 1.3, respectively. (C) Dissociation constants (K ds) for MAPK interactions with GST-Ste7 and GST-Ste5. The mean K d (in micromolar) in shown, as well as the SEM (s.e.) and the number of experiments performed to determine the mean (N). (D) The Ste7 D-site competes with Ste5241-336 for Fus3 binding. 35S-labeled, wild-type Fus3 protein (∼1 pmole) was incubated with 6 μg of purified GST or GST-Ste5241-336 prebound to glutathione-Sepharose beads, and with either no peptide, 25 or 100 μM of Ste72-22 peptide (Ste7ds) or 25 or 100 μM of a scrambled control for this peptide (scram7; (Bardwell et al., 2001)). Other details as in B, above. The bar graph shows the quantification of relative binding, as determined using a phosphorimager. After subtracting the nonspecific background adsorption to GST alone, 19.7% of the input Fus3 bound to wild-type GST-Ste5 in the absence of peptide. Results were normalized by setting this value as 100%. Data show the mean ± SD of two experiments.