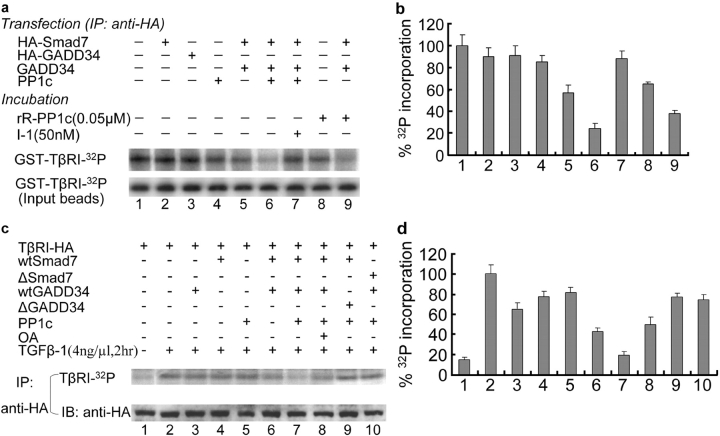
Figure 4.
Dephosphorylation of TβRI by Smad7-recruited PP1 complex. (a and b) In vitro dephosphorylation assay. (a) GST–TβRI was phosphorylated by an in vitro phosphorylation reaction. GST–TβRI–32P was incubated with different immunoprecipitates (anti-HA) from lysates of COS1 cells transfected with different combinations of genes, in the absence or presence of phosphatase inhibitor (I-1) or recombinant rabbit PP1c (rR-PP1c) as indicated. (b) The relative 32P phosphorylation level of the type I receptor in a, normalized to input of GST–TβRI–32P, is plotted as the mean ± SD from three experiments. (c and d) In vivo dephosphorylation assay. (c) Mv1Lu cells transfected with different combination of genes were labeled with [32P]orthophosphate in the presence or absence of TGFβ-1 as indicated. TβRI–HA was immunoprecipitated from lysates of treated cells and separated by 8.5% SDS-PAGE. Gels were dried and exposed to Biomax M r film (Eastman Kodak). ΔGADD34 is a mutant without the PP1c binding domain and ΔSmad7 is absent in its TβRI binding site. OA is an inhibitor for both PP1 and PP2. (d) The relative 32P phosphorylation level of TβRI in c was plotted as the mean ± SD from three experiments.