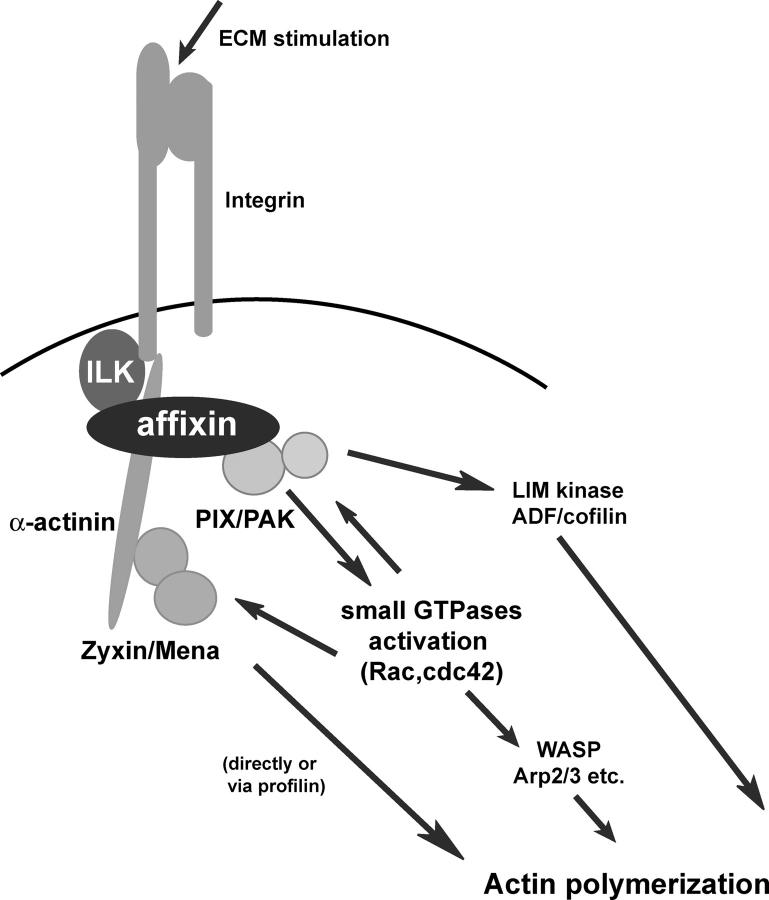
Figure 10.
Schematic illustration of the role of affixin. The integrin–ECM interaction acutely activates ILK, which is considered to induce phosphorylation of the COOH-terminal CH domain of affixin. It triggers the interaction between affixin and α-actinin, which promotes the recruitment of α-actinin into these nascent FAs. Zyxin and its binding partner, Mena, are recruited into FAs by their interaction with α-actinin. On the other hand, the NH2-terminal CH domain of affixin is considered to transmit integrin–ILK signals to activate Cdc42/Rac1 through interaction with αPIX, which results in the recruitment of PAK to FAs. Affixin-mediated formation of the signaling complex at nascent FAs should cooperatively promote actin polymerization and lead to cell spreading.