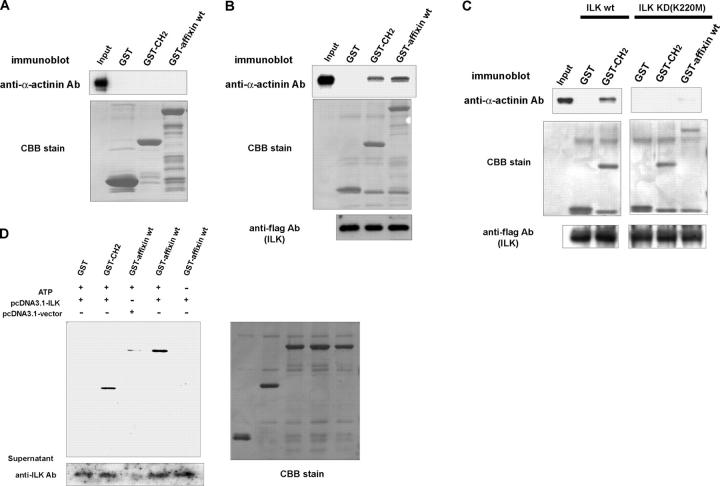
Figure 3.
Affixin requires kinase activity of ILK to directly bind to α-actinin in vitro. (A) GST full-length affixin (GST-affixin wt) or COOH-terminal CH domain (affixin213–364) of affixin fusion protein (GST-CH2) was incubated with 20 μg purified chicken α2-actinin. Coprecipitated proteins were collected using glutathione-Sepharose, resolved by SDS-PAGE, and transferred to a PVDF membrane. The membrane was probed with the anti-α-actinin antibody (top) and stained with Coomassie brilliant blue (CBB) to confirm the amount of GST fusion proteins. (B and C) These affixin fusion proteins were preincubated with protein G–Sepharose with flag antibody immunoprecipitates from lysates of Cos-7 cells overexpressing flag-tagged ILK (B) or its kinase-dead mutant (C) before incubation with α-actinin. These GST fusion proteins were similarly processed as described in A. The membrane was probed with the anti-α-actinin antibody (top) and anti-flag pAb (bottom). (D) The GST-affixin specifically interacts with α-actinin after preincubation with ILK. GST-affixin wt, GST-CH2, or GST alone was separated by SDS-PAGE after preincubation with in vitro–translated ILK in the presence or absence of 10 μM ATP in phosphorylation buffer, and was transferred to membrane. Blot overlay assay was done using 10 μg/ml purified α-actinin after blocking.