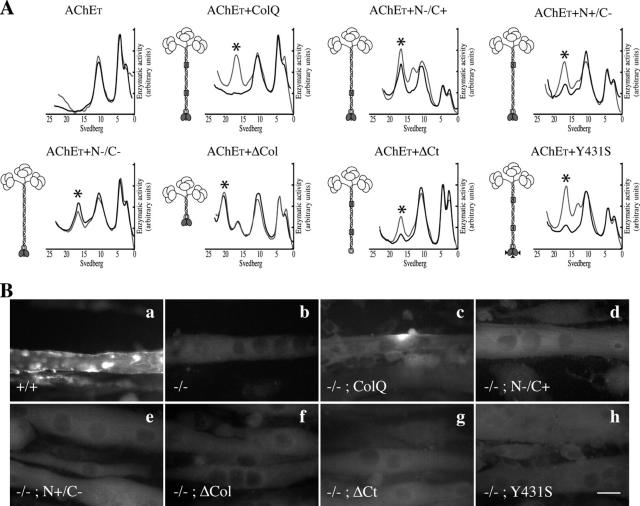
Figure 2.
Distinct sites of ColQ are necessary for the formation of AChE clusters. (A) Analysis of AChE molecular forms secreted from the ColQ-deficient myogenic cells transfected with AChET or cotransfected with AChET and wt ColQ or ColQ mutants. Sedimentation profiles of the molecular forms are shown in black lines. Asterisk indicates the position of A12 forms in the sedimentation profiles. Co-transfected cells were also treated for 24 h by heparin. Sedimentation profiles of the molecular forms secreted after heparin treatment are shown in gray lines. A schematic representation of AChE A forms and ColQ mutant constructs used in this work are shown on the left side of the gradients. Catalytic subunits (ovoids) are associated to a triple helix of ColQ. This last protein contains a collagen domain (186–285 aa) flanked by a NH2-terminal peptide (1–186 aa) and a COOH-terminal peptide (285–450 aa). The two HBS (N+ and C+) contained in the collagen domain are indicated as gray boxes. In mutant construct N−/C+, RK (121–122 aa) is mutated to DP. In mutant construct N+/C−, KR (226–227 aa) is mutated to DP. In construct N−/C−, both sites are mutated. In mutant ΔCol, amino acids 105–276 were deleted. In mutant ΔCt, amino acids 362–450 were deleted. The rat Y431S construct reproduces the missense mutation detected in the human ColQ COOH terminus domain. As shown, none of the mutations prevented the formation and secretion of AChE A forms. (B) Cells were labeled with antibodies to AChE. Wt myogenic cells (+/+) formed AChE clusters (a), whereas no AChE clusters were detected in myogenic cells derived from ColQ-deficient mice (−/−; b). ColQ transfected in ColQ-deficient muscle cells restored the formation of AChE clusters (c), but no AChE clusters were detected when these cells were transfected with either the N−/C+ construct (d), the N+/C− construct (e), the ΔCol construct (f), the ΔCt construct (g), or the Y431S construct (h).