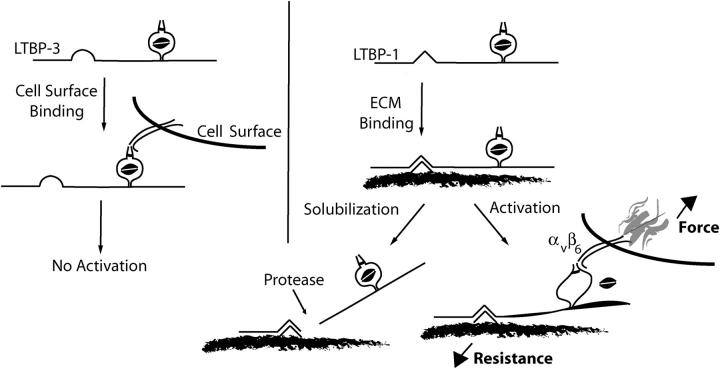
Figure 8.
Schematic representation of αVβ6-mediated latent TGF-β activation. TGF-β is secreted in a complex with a variety of LTBP isoforms and splice variants. The highly variable primary sequence of the hinge domain localizes latent TGF-β in the extracellular environment. Importantly, the hinge domain of LTBP-1 functions in a capacity that is not replicated by the hinge domain of LTBP-3. Once latent TGF-β is fixed in the ECM, the integrin αVβ6 binds LAP and generates a retractile force. The magnitude of this force is related to the resistance garnered through association of the latent complex with the ECM. Once the force generated by the integrin exceeds a threshold, biologically active TGF-β is made available. Release of the latent complex from its association with the ECM, for example by proteases, is predicted to prevent αVβ6-mediated latent TGF-β activation as integrin retraction will no longer be resisted.