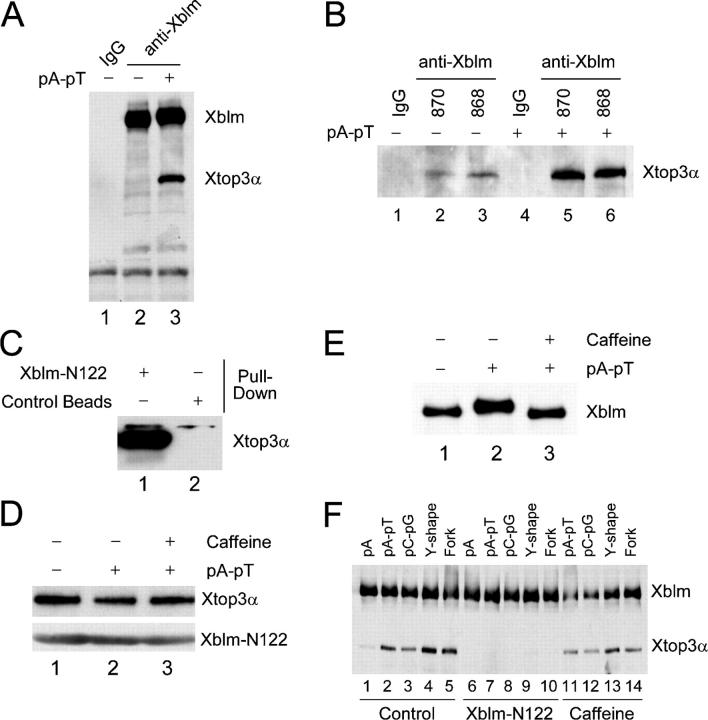
Figure 4.
Xblm interacts with Xtop3α in a regulated manner. (A) Control (lane 1) and anti-Xblm (lanes 2 and 3) antibodies on protein A beads were incubated in extracts in the absence (lanes 1 and 2) or presence (lane 3) of pA-pT. The beads were collected and immunoblotted for Xblm and Xtop3α. (B) Control antibodies (lanes 1 and 4) and two different anti-Xblm antibodies (#870, lanes 2 and 5; #868, lanes 3 and 6) on protein A beads were incubated in extracts in the absence (lanes1–3) or presence (lanes 4–6) of pA-pT. The beads were collected and immunoblotted for Xtop3α. (C) His6-tagged Xblm-N122 on nickel beads and blank nickel beads were incubated in interphase egg extracts. The beads were collected and immunoblotted for Xtop3α. (D) Xblm-N122 on nickel beads was incubated in extracts containing no DNA (lane 1), pA-pT (lane 2), or pA-pT plus caffeine (lane 3). The beads were collected and immunoblotted with anti-Xtop3α and anti-His6 antibodies (to detect Xblm-N122). (E) Egg extracts were incubated with no DNA (lane 1), pA-pT (lane 2), or pA-pT plus caffeine (lane 3) and were immunoblotted for Xblm. (F) Anti-Xblm antibodies on protein A beads were incubated in extracts in the presence of different oligonucleotides. In some cases, either purified Xblm-N122 (lanes 6–10) or caffeine (lanes 11–14) was also added. After 2 h, the beads were collected and immunoblotted for Xblm and Xtop3α.