Figure 1.
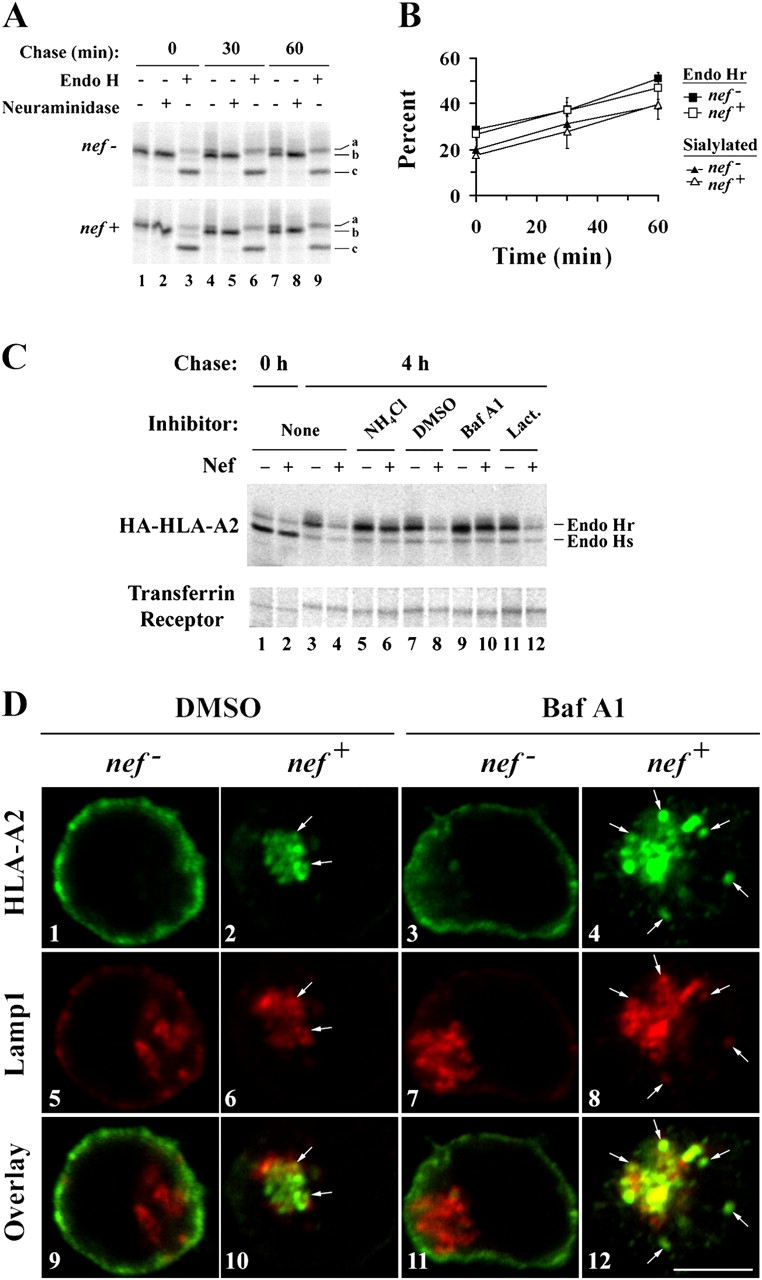
HIV-1 Nef redirects MHC-I from the TGN to lysosomes. (A and B) Nef does not affect the maturation of HLA-A2 through the TGN. CEM HA-HLA-A2 cells were transduced with a control adenovirus (nef −) or an adenovirus expressing HIV-1 Nef (nef +) and subjected to a pulse-chase metabolic labeling assay to follow proteins through the biosynthetic pathway. HA-HLA-A2 species with different gel mobilities were identified based on enzymatic digestion profiles: (a) N-glycosylated and sialylated HA-HLA-A2; (b) N-glycosylated HA-HLA-A2; and (c) core HA-HLA-A2 protein. (B) Quantitation of the relative percentage of endo H resistant and sialylated HA-HLA-A2. The mean percentage ± SD from two independent experiments is plotted over time. (C) Nef-induced degradation of mature MHC-I is blocked by inhibitors of acidic degradation. CEM HA-HLA-A2 cells were treated with adenovirus, pulsed with radioactive amino acids, and were either collected immediately (lanes 1 and 2) or chased for 4 h in media containing the indicated chemical inhibitor (lanes 3–12). Cellular lysates were divided equally, and HA-HLA-A2 or transferrin receptor was recovered by immunoprecipitation. All samples were digested with endo H before SDS-PAGE. The results are representative of three independent experiments. (D) Bafilomycin A1 treatment increases the degree of colocalization between HLA-A2 and LAMP-1. Cells transduced with the indicated adenovirus were treated with either solvent alone (DMSO) or bafilomycin A1 (Baf A1) for 4 h before staining. HLA-A2 and LAMP-1 were detected by indirect immunofluorescence using mAbs as described in Materials and methods. Arrows indicate colocalization between HLA-A2 and LAMP-1. Images were collected using a confocal microscope. Individual z-sections are shown. Bar, 5 μm.
