Figure 4.
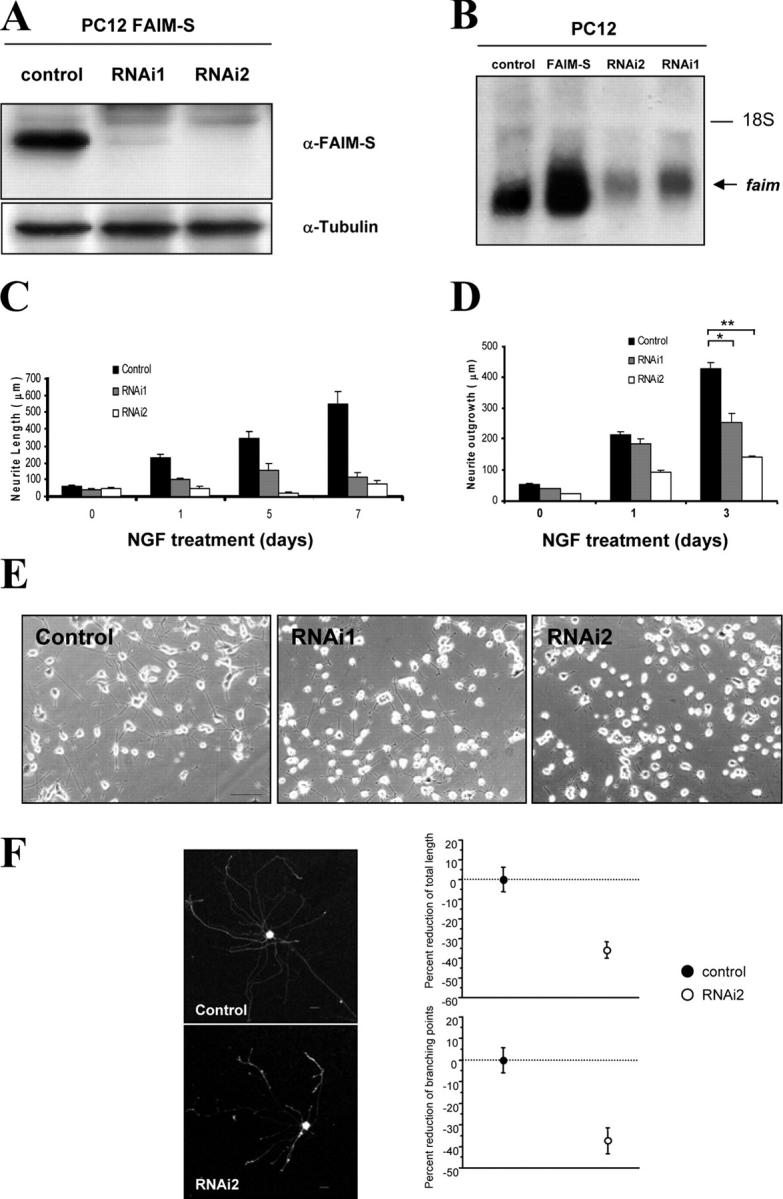
FAIM-S is necessary for NGF-induced PC12 cells differentiation. (A) PC12 cells that stably express FAIM-S were transfected with pSUPER containing sequences encoding RNAi against FAIM (RNAi1 or RNAi2) or the empty vector (control). Stable pools of transfected cells were obtained with puromycin. Extracts were probed with the anti-FAIM antibody by Western blot analysis (top). Membranes were stripped and reprobed with an antibody to α-tubulin used to control the loading of lanes. (B) PC12 cells were stably transfected with an empty vector (control), with the FAIM-S expression plasmid, or with the plasmid RNAi1 or RNAi2. Total RNA (15 μg) was extracted, electrophoresed, and hybridized with a [32P]dCTP-labeled FAIM probe. Images were obtained by autoradiography for 40 h at −80°C. (C) PC12 cells were transiently transfected with eYFP and either RNAi1 or RNAi2, or with the empty vector (control). After 4 d, cells were treated with 100 ng/ml NGF for 1 d. Histogram shows the neurite length measurements of the labeled and digitally acquired cultures transfected with the indicated cDNA. (D and E) PC12 cells were stably transfected with either an empty vector (control) or with either of the two FAIM RNAi (RNAi1 and RNAi2). (D) Cells were treated with NGF for 1 or 3 d or left untreated and total neurite length was measured. (E) Representative images of NGF-induced differentiation on stably transfected cells after 1 d of treatment. Bar, 100 μm. (F) Rat SCG neurons were cotransfected with eYFP and either the empty pSUPER (control) or the vector containing RNAi2. After 60 h of NGF treatment (10 ng/ml), eYFP-labeled SCG were visualized, digitally acquired (representative images are shown on the left), and neurite outgrowth was quantified. Neurite length (top) and the branching points (bottom) were quantified. Bars, 25 μm.
