Figure 4.
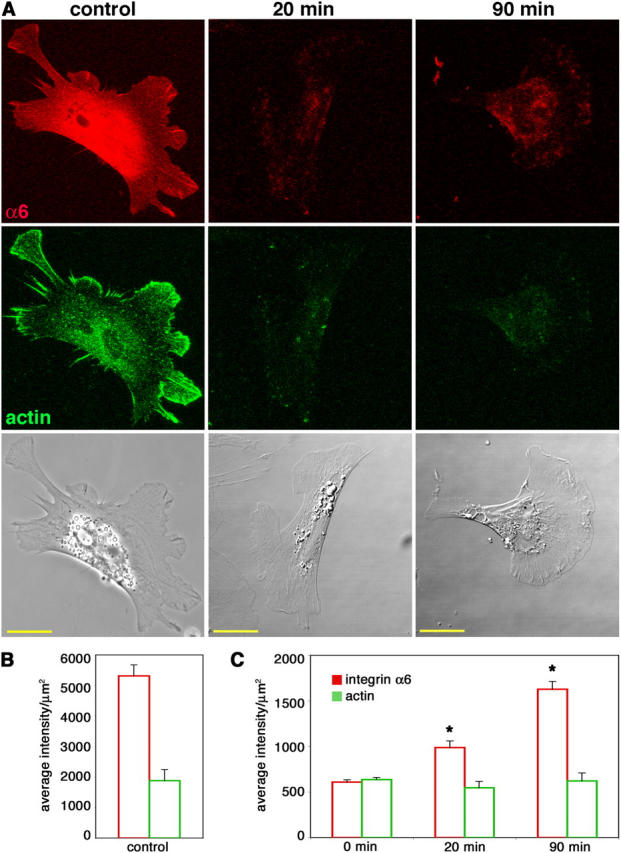
Cranial NCCs recycle internalized integrin α6 back to the cell surface. Cells cultured on high laminin concentrations were allowed to internalize anti-integrin α6 Fab bound to surface receptors for 30 min. The remaining cell surface Fab was blocked and cells were reincubated at 37°C for 20 or 90 min to allow for exocytosis of the integrin α6–Fab complex. (A) Recycled integrin α6 was detected by labeling the unblocked Fab that had reappeared on the surface (red). Cells were also stained for actin (green) as a permeabilization control. Control cells were intentionally permeabilized to show total integrin α6 and actin staining. Bars, 20 μm. (B and C) Average intensities per unit area + SEM (arbitrary units) of integrin α6 and actin fluorescence were determined for at least 20 cells from three independent experiments for each condition. (B) Measurements from permeabilized control cells, as shown in the first column in A, have significantly higher integrin α6 and actin fluorescence than unpermeabilized 0-min controls, as shown in C (P < 0.001; t test). (C) In unpermeabilized cells, actin fluorescence is constant, whereas integrin α6 fluorescence increases at both 20 and 90 min. Asterisk indicates significant difference from 0-min levels (P < 0.0001; t test).
