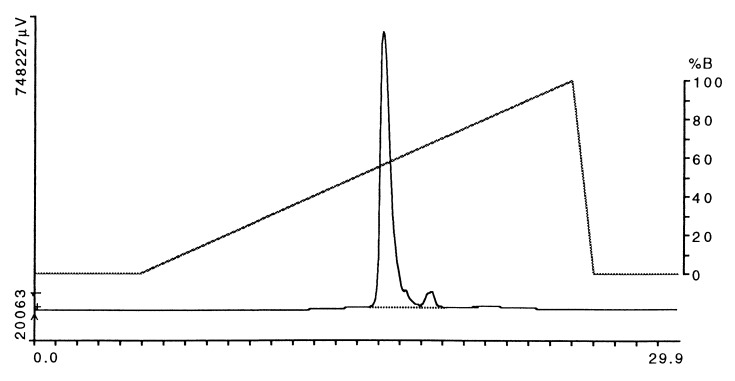
Figure 3.
Purification of the HLA-DR2/MBP peptide complex by anion-exchange HPLC after cleavage of leucine zipper dimerization domains. After affinity purification using mAb L243, the leucine zipper dimerization domains were cleaved with V8 protease (using 5 μg of enzyme per mg of DR2/MBP peptide complex). The HLA-DR2/MBP peptide complex was further purified by using a DEAE column (Mono Q, Pharmacia). Six milligrams of protein was loaded onto the column followed by elution with a 20-min linear NaCl gradient (buffer A, 50 mM ethanolamine, pH 9.0/0.02% sodium azide; buffer B, 50 mM ethanolamine, pH 9.0/0.02% sodium azide/1 M NaCl). The major peak that eluted between ≈55% and 60% B was used for crystallization. Horizontal axis, time (min); left vertical axis, absorbance at 280 nm, expressed in μV.