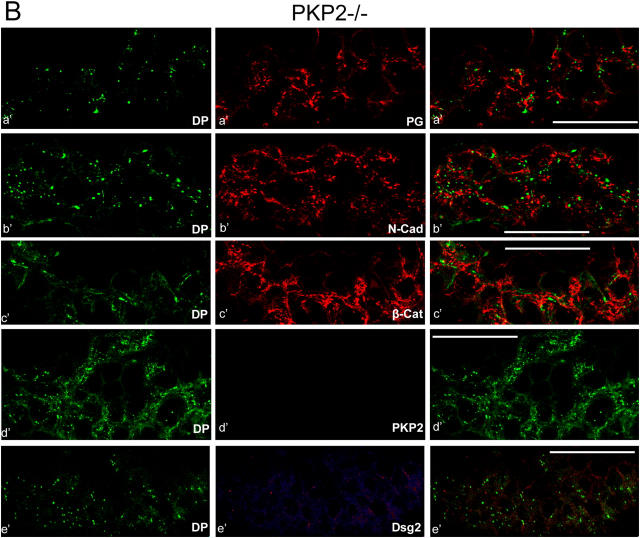
Figure 4.

Major architectural and compositional changes of the adhering junctions in the intercalated disks of hearts in plakophilin 2–deficient E10.75 mouse embryos. Laser-scanning, double-label immunofluorescence microscopy of cryostat sections through cardiac tissue, performed with wt and plakophilin 2–deficient (pkp2−/−) embryos. Specific combinations of antibodies were used: rabbit antibodies to desmoplakin (DP, marked by green fluorescence) and murine mAbs to diverse other cardiac adhering junction components (red fluorescence): (a and a′) plakoglobin (PG); (b and b′) N-cadherin (N-Cad); (c and c′) β-catenin (β-Cat); (d and d′) plakophilin 2 (PKP2); (e and e′) desmoglein 2 (Dsg2). The merged fluorescence images are shown in the right-hand column. (A) In the wt embryos, the special adhering junctions of the intercalated disks show far-reaching colocalization (yellow) of desmoplakin with (a) plakoglobin, (b) N-cadherin, (c) β-catenin, (d) plakophilin 2, and (e) desmoglein 2. Other known plaque proteins of cardiac adhering junctions show identical localization, including p120ctn and α-catenin (see also Janssens et al., 2001). (B) By contrast, in the pkp2−/− embryos none of the plaque proteins colocalize with desmoplakin (a′–e′). In the mutants, desmoplakin is dispersed over the cytoplasm, often appearing in granular aggregates (green dots). (d′) Complete absence of plakophilin 2 immunostaining in the mutant embryos is shown. (e′) Note that Dsg2 is not detectably enriched in the intercalated disks of the mutants. Bars, 50 μm.