Figure 1.
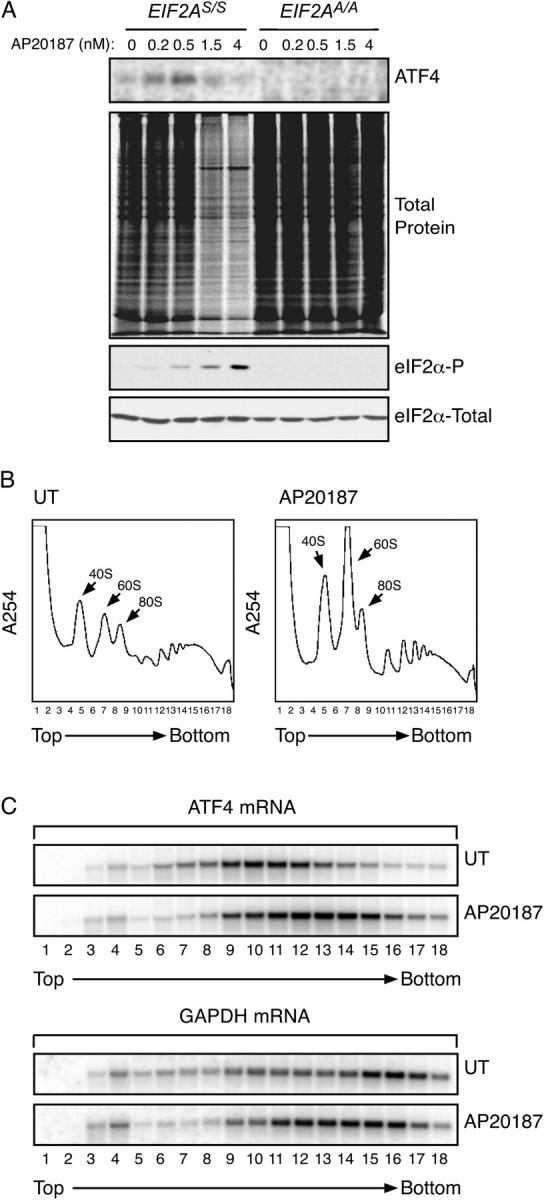
EIF2α phosphorylation is sufficient to up-regulate ATF4 translation. (A) Autoradiogram of SDS-PAGE of radiolabeled proteins after a brief labeling pulse of Fv2E-PERK expressing wild-type (EIF2A S/S) or mutant (EIF2A A/A) mouse fibroblasts pretreated for 30′ with the indicated concentration of the AP20187 activating ligand. The panels (from top to bottom) show immunoprecipitated radiolabeled endogenous ATF4, all radiolabeled newly synthesized proteins, and immunoblots of phosphorylated and total eIF2α. (B) Polysome profiles of mRNA isolated from untreated mouse fibroblasts and the same cells 60′ after induction of eIF2α phosphorylation by activation of the ligand-inducible Fv2E-PERK eIF2α kinase. Note the accumulation of ribosomal subunits and monosomes in the treated cells. (C) Northern blot analysis of ATF4 and GAPDH mRNA from fractions collected from the gradient shown in B. Note the shift to the right, (heavier) fractions in the peak of the ATF4 mRNA in the treated cells and the shift in opposite direction of the GAPDH peak.
