Figure 2.
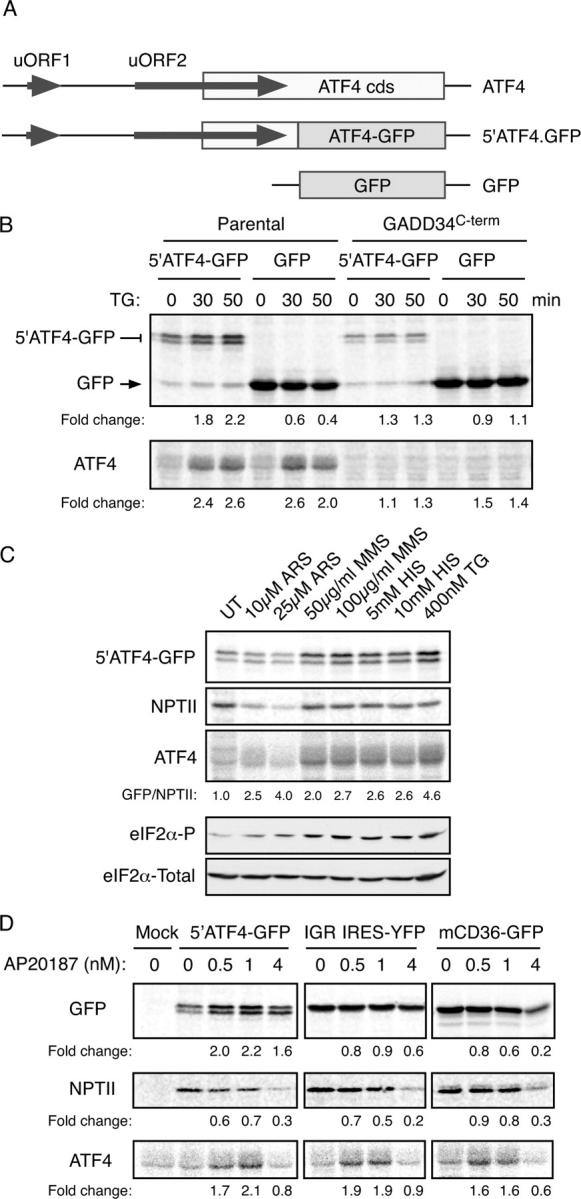
The 5′ end of the ATF4 mRNA mediates translational regulation of the gene. (A) Organization of the 5′ end of the mouse ATF4 mRNA, the derivative 5′ATF4-GFP reporter and the parental GFP reporter. (B) Autoradiogram of SDS-PAGE of radiolabeled proteins after a brief labeling pulse of wild-type CHO cells or cells stably overexpressing the COOH terminus of GADD34 (which blocks eIF2α phosphorylation) transfected with the indicated reporter plasmids. The cells were treated with the endoplasmic reticulum stress-inducing agent thapsigargin (TG) for the indicated time and the encoded GFP fusion proteins (top) and endogenous ATF4 (bottom) were immunoprecipitated with specific antibodies. (C) Autoradiogram of SDS-PAGE of radiolabeled proteins after a brief labeling pulse of CHO cells transfected with the ATF4-GFP reporter and pretreated for 30 min with the indicated concentration of arsenite (ARS), the electrophile methyl-methanesulfonate (MMS), histidinol (HIS, which activates GCN2), thapsigargin (TG), and the encoded GFP fusion proteins (top), the NPTII, encoded by a different gene on the same plasmid (second panel) and endogenous ATF4 (third panel) were immunoprecipitated with specific antisera. The GFP/NPTII ratio provides an estimate of the translational inducibility of the reporter in the treated cells. Immunoblots of phosphorylated (eIF2α-P) and total eIF2α from parallel lysates are shown in the bottom panels. (D) Autoradiogram of radiolabeled proteins from Fv2E-PERK expressing CHO cells treated with the indicated concentration of the activating AP20187 ligand for 30′ before and during the 20′ labeling pulse. The cells were transfected with the ATF4 reporter (5′ATF4-GFP), a cricket paralysis virus internal ribosome entry site reporter (IGR IRE5-YFP) or a GFP translational reporter derived from the 5′ end of mouse CD36 gene (mCD36-GFP). GFP, endogenous ATF4 and NPTII were immunoprecipitated as in C with specific antisera.
