Figure 8.
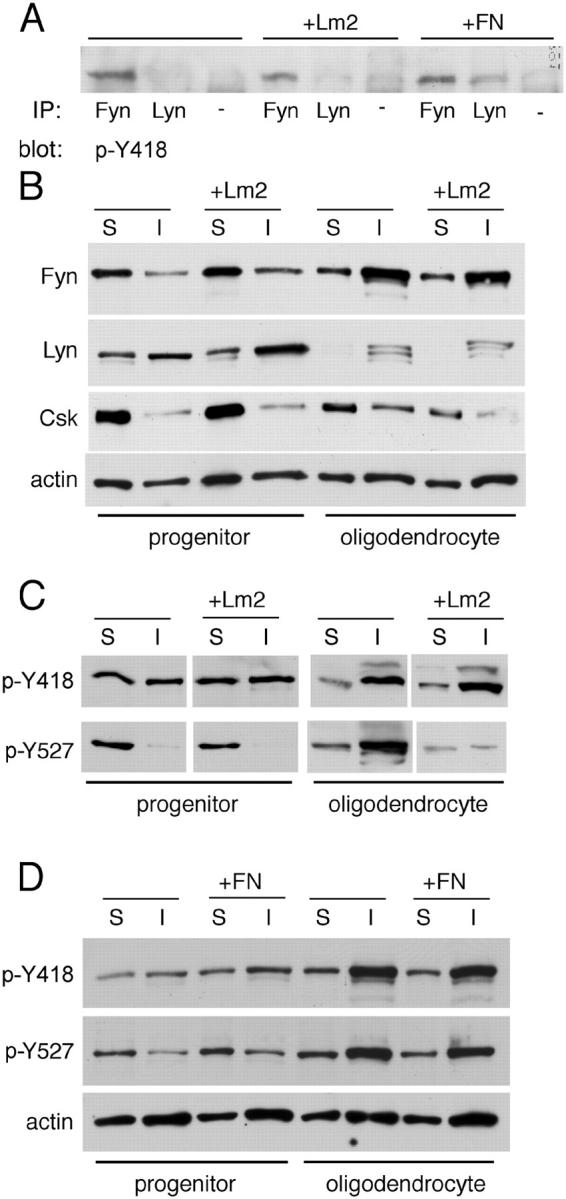
SFK activity is regulated by laminin in differentiating oligodendrocytes, not progenitors. (A) Active Lyn is detected in response to the αVβ3 ligand FN. Immunoprecipitation complexes using antibodies against Fyn, Lyn, or control mouse IgG (−) were evaluated by Western blot for the presence of autophosphorylated SFK phosphoY418. (B) Expression and solubility of Fyn, Lyn, and COOH-terminal Src kinase (Csk) change during oligodendrocyte lineage progression and in response to Lm2. Western blots were performed using antibodies specific for SFKs and SFK regulatory kinase Csk. S, Triton X-100–soluble protein; I, Triton X-100–insoluble protein. Blots were also probed with actin antibodies as protein loading controls. (C) Phosphorylation of the SFK negative regulatory site is reduced by laminin in oligodendrocytes, not progenitors. The same lysates as in B were used, but Western blots were analyzed using antibodies against two SFK sites: phosphoY418 (catalytic) and phosphoY527 (COOH-terminal negative regulatory). (D) FN does not alter phosphorylation of the SFK negative regulatory site. Lysates of progenitors and oligodendrocytes grown on control substrate PDL or on the αVβ3 ligand FN. Western blots were performed using SFK antibodies specific for phosphoY418 (catalytic) and phosphoY527 (COOH-terminal negative regulatory). Blots were also probed with actin antibodies as protein loading controls. (A–D) In the absence of ECM ligands, cells were grown on non-integrin substrate PDL.
