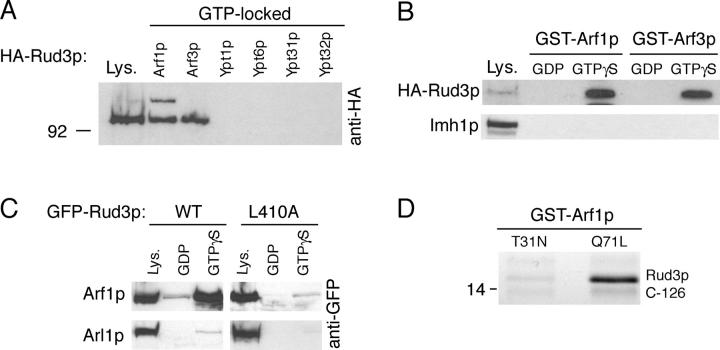
Figure 3.
Rud3p interacts with the small GTPase Arf1p. (A) Anti-HA protein blot of total cell lysate (Lys.) from a strain expressing Rud3p tagged in the genome with an NH2-terminal HA epitope tag (AGY10) and of proteins that bound to GST fusions of the GTP-locked versions of Arf1p, Arf3p, Ypt1p, Ypt6, Ypt31p, and Ypt32p. For the GTP-locked forms of Arf1p (Q71L) and Arf3p (Q71L), the first 14 amino acids that form an amphipathic helix were removed and replaced with an NH2-terminal GST tag. For Ypt1p (Q67L), Ypt6p (Q67L), Ypt31p (Q72L), and Ypt32p (Q72L), the COOH-terminal cysteine residues were replaced with a COOH-terminal GST tag. Lysate is 10% of material applied to beads. (B) Anti-HA protein blot of total cell lysate (Lys.; 10% of material loaded) and proteins that bound to GST fusions of wild-type Arf1p and Arf3p preloaded with GDP or a nonhydrolysable analogue of GTP, GTPγS. The blot was stripped and reprobed with a rabbit antibody against Imh1p. (C) As for GST-Arf1p in B, except cell lysates were prepared from a strain expressing either GFP-Rud3p or the mutant proteins L410A expressed under the control of a constitutively active PHO5 promoter from the CEN plasmid pRS416. (D) Binding of the COOH-terminal 126 amino acids of Rud3p to Arf1p (T31N) or Arf1p (Q71L). The indicated forms of GST-Arf1p were coexpressed in E. coli with the COOH terminus of Rud3p, and after cell lysis isolated on glutathione Sepharose beads. Bound proteins were analyzed by gel electrophoresis, and the indicated band was identified as the COOH terminus of Rud3p by matrix-assisted laser desorption ionization mass spectrometry of tryptic peptides (Shevchenko et al., 1996).