Figure 7.
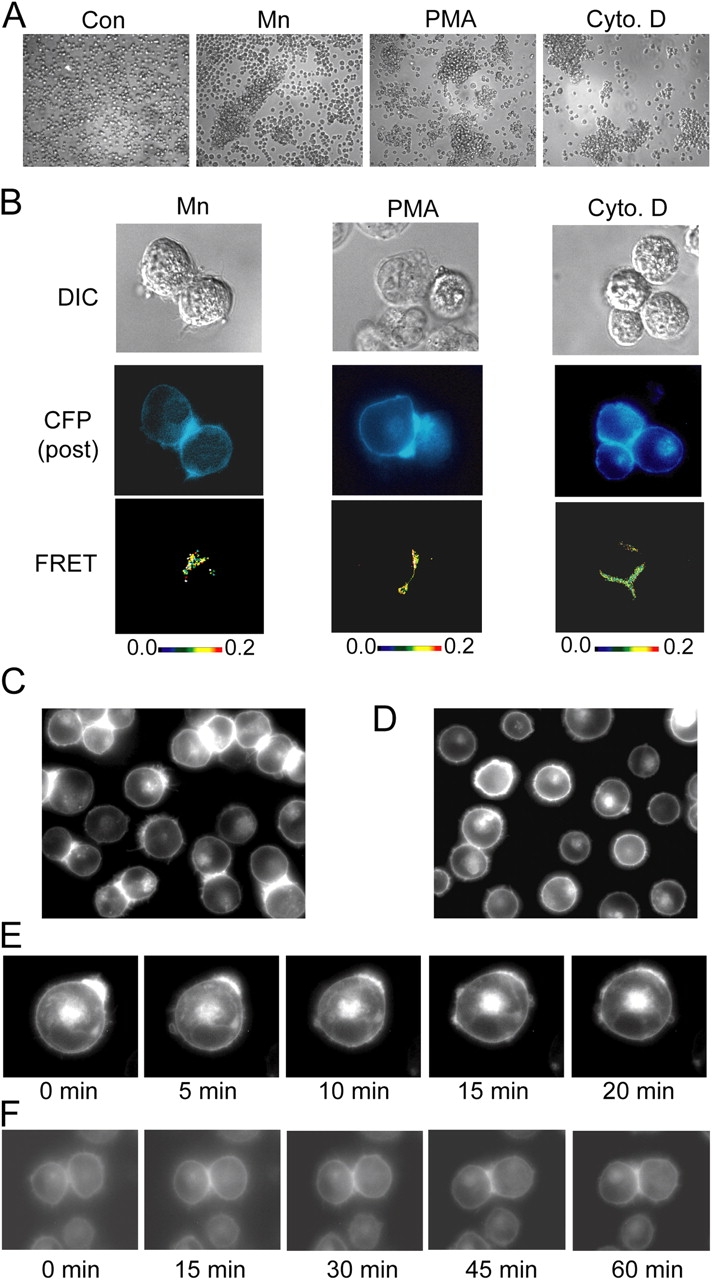
Homotypic cell aggregation promotes LFA-1 macro- and microclustering at the cell–cell contact interface. (A) K562 transfectants expressing wild-type αLβ2 in 1.5-ml microfuge tubes (106 cells in 100 μl L15 medium + 2 mg/ml glucose) were centrifuged at 200 g for 30 s and incubated in the absence (control) or presence of 1 mM Mn2+, 1 μM PMA, or 1 μM cytochalasin D for 1 h at 37°C, transferred to cell culture dishes, and imaged by phase-contrast microscopy using a 10× objective in the original experiment. (B) Transient αL-mCFP/β2 and αL-mYFP/β2 K562 transfectants were treated exactly as in A, and inter-heterodimer FRET was measured for homotypically adherent cells. DIC (top), CFP fluorescence after YFP bleaching (middle), and pixel-by-pixel FRET efficiency (from 0 [black] to 0.2 [red]) (bottom) are shown. (C and D) K562 transfectants stably expressing αL-mCFP/β2-mYFP and the talin head domain (Kim et al., 2003) were gently removed from culture flasks and either directly plated on cover glasses and imaged with YFP fluorescence (C) or micropipetted 5–10 times to obtain single suspensions, plated, and incubated for 10 min before imaging YFP fluorescence (D). (E) Aggregates of K562 transfectants expressing αL-mCFP/β2-mYFP and the talin head domain were dissociated as in D and immediately subjected to time-lapse fluorescence imaging at 37οC. (F) To observe the formation of macroclusters, cells treated as in D were allowed to reestablish homotypic cell–cell contacts during time-lapse fluorescence imaging. Images are from representative experiments. (See also Video 2 and Video 3, available at http://www.jcb.org/cgi/content/full/jcb.200404160/DC1).
