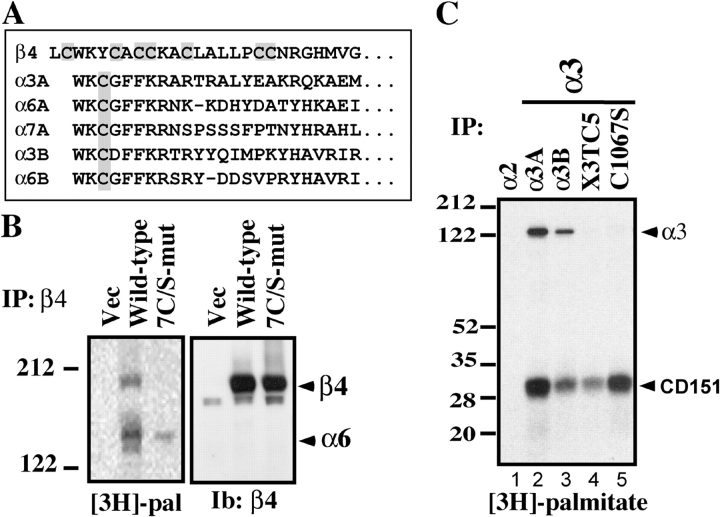
Figure 3.
Identification of integrin palmitoylation sites. (A) Membrane-proximal regions containing candidate palmitoylation sites are shaded gray. The first lysine defines a putative transmembrane interface. A and B designate alternatively spliced forms of integrin cytoplasmic tails. (B) MDA-MB-435 cells stably expressing mutant or wild-type β4, or vector control, were [3H]palmitate labeled and lysed in 1% Brij 96. The α2 integrin was immunoprecipitated from vector control cells, and α6β4 was immunoprecipitated (using anti-α6 mAb GoH3) from β4-transfected cells. Shown are proteins labeled with [3H]palmitate (left) or blotted with anti-β4 antibody (right). (C) Murine B12 cells, stably expressing human integrin α subunits, were [3H]palmitate labeled and lysed in 1% RIPA. Integrins were immunoprecipitated using anti–human α2 (lane 1) and α3 (lanes 2–5) antibodies (A2-IIE10 and A3-X8) and resolved by SDS-PAGE, and [3H]palmitate was detected. The α3 subunits include α3-X3TC5 (α3 tail and transmembrane regions are replaced by those regions from α5; Yauch et al., 1998) and α3-C1067S (palmitoylation site point mutant).