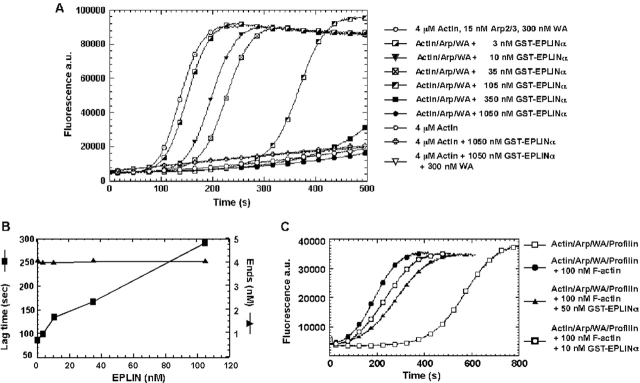
Figure 6.
EPLIN inhibits actin nucleation by Arp2/3 complex. (A) The time course of actin polymerization was monitored by pyrene fluorescence. Samples contained 4 μM G-actin (5% pyrene labeled), ± 15 nM Arp2/3 complex, or ± 300 nM Scar-WA domain, ± varying concentrations of GST-EPLIN-α in polymerization buffer. (B) Dependence of the lag time and concentration of ends produced by Arp2/3 complex on the concentration of EPLIN-α. The polymerization lag time was defined as the time required to achieve 10% of the maximum fluorescence. The concentration of barbed ends was calculated from elongation rate (measured by the rate of polymerization where 80% of monomers were polymerized) using the equation: [barbed ends] = elongation rate/(k + [actin monomer]), where k + = 10 μM−1 s−1. At GST-EPLIN-α concentrations >105 nM, polymerization was incomplete in the time course of the experiment, disallowing determination of the lag time or the concentration of barbed ends. (C) The effect of EPLIN on the ability of preformed actin filaments to stimulate nucleation by Arp2/3 complex. Samples in polymerization buffer contained 2 μM G-actin (5% pyrene labeled), 15 nM Arp2/3 complex, 300 nM Scar-WA domain, and 8 μM profilin-I, ± 100 nM actin filament, ± 10 or 50 nM GST-EPLIN-α. Profilin-I was added to accentuate the polymerization lag time.