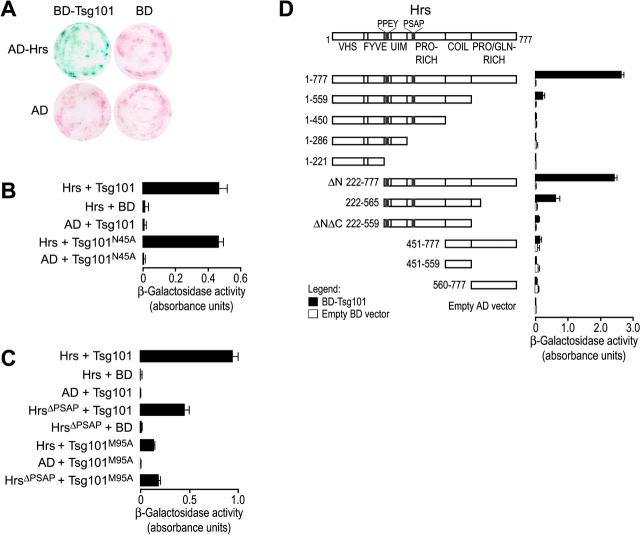
Figure 3.
Hrs/Tsg101 yeast two-hybrid binding assays. (A) Filter paper colony lift assay for β-galactosidase reporter gene activity. Yeast cells were cotransformed with pairs of plasmids expressing the indicated Gal4 DNA-binding domain (BD) and activation domain (AD) fusion constructs. Cells were grown on synthetic agar media lacking the appropriate amino acids and were analyzed for β-galactosidase activity (blue) as described in Materials and methods. (B) Binding of Hrs to wild-type and mutant (N45A) Tsg101. Binding experiments in B–D show yeast two-hybrid interactions (together with appropriate controls) as measured in semi-quantitative CPRG β-galactosidase activity assays. Bars depict the average absorbance (595 nm) and SDs from three independent measurements. (C) Binding of wild-type and mutant (ΔPSAP) Hrs to wild-type and mutant (M95A) Tsg101. (D) Binding of Tsg101 to Hrs deletion mutants.