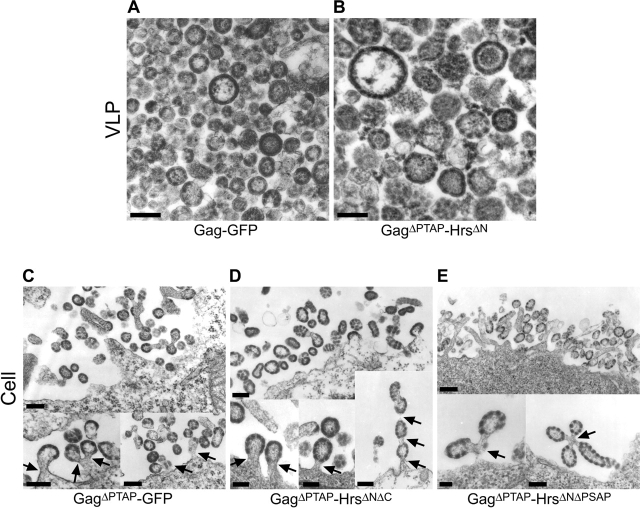
Figure 5.
Electron microscopic analyses of Gag–GFP and Gag–Hrs budding. (A and B) Transmission electron micrographs of thin-sectioned VLPs released from 293T cells transfected with plasmids encoding Gag–GFP (A) and GagΔPTAP–HrsΔN (B). Note that in both cases, the proteins formed enveloped, spherical VLPs that resembled authentic immature virions except that (1) the electron-dense Gag layer beneath the VLP membrane often appeared discontinuous, suggesting that the COOH-terminal GFP and Hrs polypeptides perturbed the underlying Gag lattice; and (2) VLPs formed by GagΔPTAP–HrsΔN exhibited greater size variation and were, on average, somewhat larger than authentic immature virions. (C–E) Transmission electron micrographs of thin-sectioned 293T cells transfected with plasmids expressing GagΔPTAP–GFP (C), GagΔPTAP–HrsΔNΔC (D), and GagΔPTAP–HrsΔNΔPSAP (E). The top images show clusters of particles associated with the cellular surface, and the bottom images show examples of classic “late domain” phenotypes in which the assembled particles remained tethered to the plasma membrane (or to one another). Bars, 100 nm.