Figure 1.
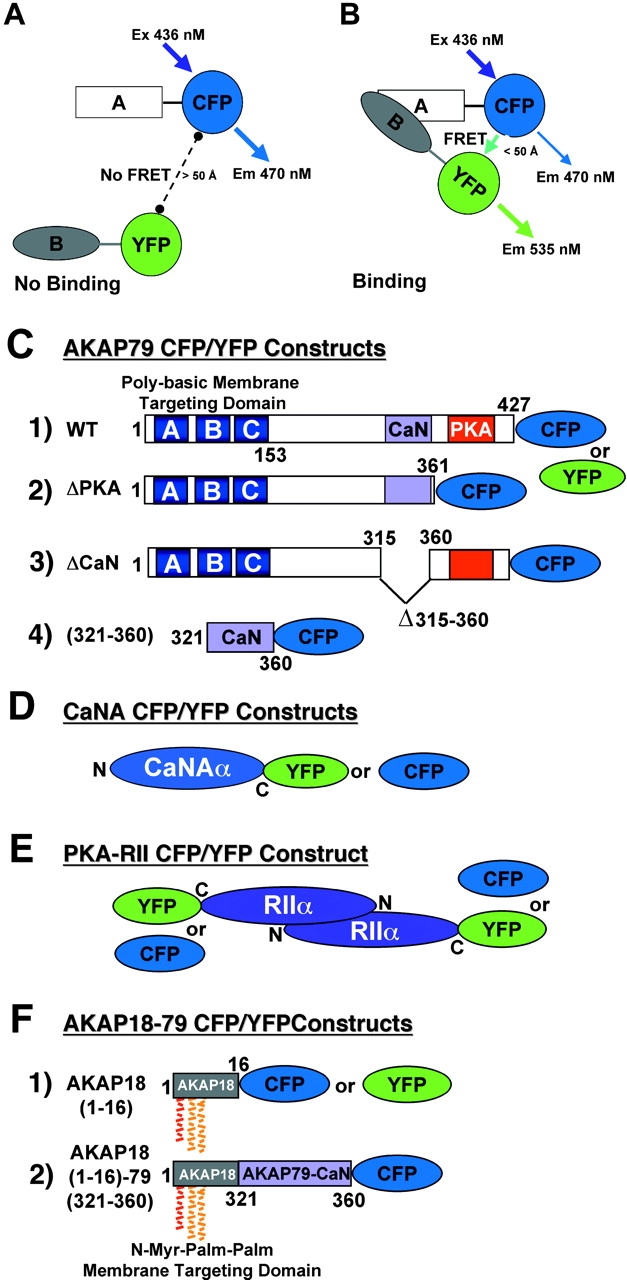
CFP/YFP FRET microscopy system for studying AKAP79 protein–protein interactions in living cells. (A) Model showing no FRET between A–CFP and B–YFP tagged proteins in the absence of binding. (B) CFP to YFP FRET (CYFRET) resulting in increased YFP acceptor emission and quenching of CFP donor emission seen when A–CFP and B–YFP tagged proteins are bound within 50 Å of each other. (C) CFP- and YFP-tagged (1) AKAP79 WT, (2) ΔPKA, (3) ΔCaN, and (4) (321–360) constructs used for CYFRET imaging of AKAP79 binding to CFP- and YFP-tagged (D) CaNAα and (E) PKA-RIIα. (F) (1) AKAP18(1–16) and (2) AKAP18(1–16)–AKAP79(321–360) hybrid CFP and YFP fusion proteins for CYFRET imaging of CaNA binding. AKAP79 anchors the CaNA,B holoenzyme through binding the catalytic CaNA subunit (Coghlan et al., 1995; Kashishian et al., 1998) and anchors the PKA heterotetrameric R2C2 holoenzyme through binding a surface created by dimerization of the of NH2 terminus of the regulatory subunit (RIIα/β) (Newlon et al., 2001). The NH2 and COOH termini of each protein are indicated by N and C or numbers starting with 1 at the NH2 terminus. The AKAP79 CaN (315–360, pink) and PKA (388–413, red) binding sites are indicated as are the AKAP79 (1–153, A, B, and C poly-basic, blue) and AKAP18 (1–16, NH2-terminal Gly-myristoylated, dual Cys-palmitoylated) membrane targeting domains.
